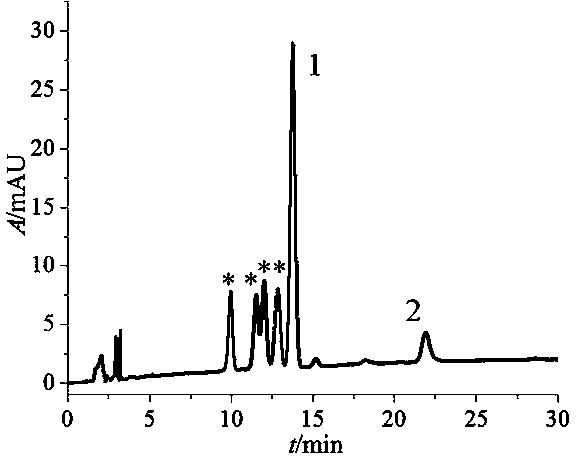
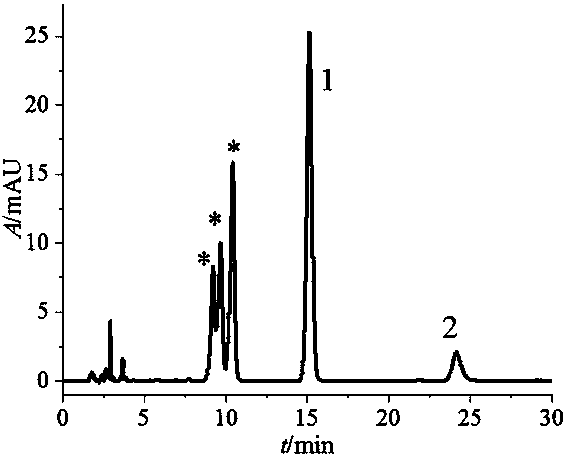
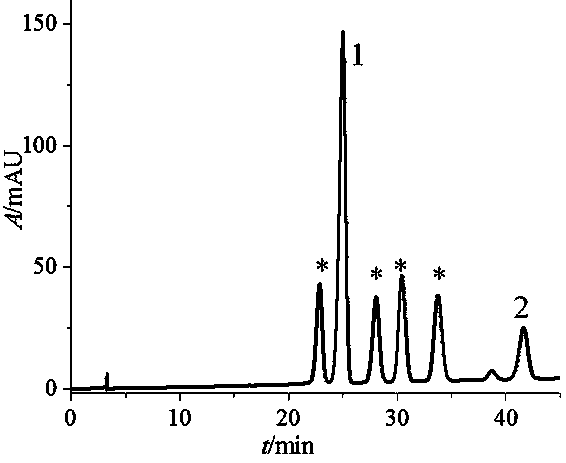
Method for preparation and purification of four stereoisomers of benzopyrene-DNA adduct
A technology of DNA adducts and stereoisomers, applied in the preparation of sugar derivatives, organic chemistry methods, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Synthesis of anti-BPDE-N 2 -dG
[0032] Weigh 0.1 mg (±)anti-BPDE and dissolve in 100 μL of tetrahydrofuran: triethylamine=19:1 solution; weigh 4.8 mg of 2'deoxyguanosine (dG) and dissolve in 1.2 mL 50 mmol / L Tris -HCl, pH=7.5 buffer solution, mix the two, avoid light, and react at room temperature at 23.6°C for 48 hours.
[0033] 2. Preliminary purification by solid phase extraction
[0034] To extract the target product anti-BPDE-N from the reaction solution 2 -dG, requires solid phase extraction for preliminary purification and enrichment, and removes the reaction base solution. Weigh 100 mg Si-C 18 Adsorbent, methanol was activated and loaded into the solid-phase extraction column, 5 mL of water was replaced by methanol, the reaction solution was passed through the activated solid-phase extraction column, and then 2 mL of ultrapure water, 1 mL of 10% methanol aqueous solution were used successively, Rinse with 1 mL of 30% methanol aqueous solution, drain the...
Embodiment 2
[0058] 1. Synthesis of anti-BPDE-N 2 -dG
[0059] Weigh 0.2 mg (±) anti-BPDE and dissolve it in 100 μL of tetrahydrofuran: triethylamine = 17:1 solution to obtain A solution, the concentration of BPDE in A solution is: 2 g / L; weigh 8.9 mg of 2'deoxyguanosine (dG) was dissolved in 1.2 mL of 50 mmol / LTris-HCl pH=7 buffer solution to obtain solution B. In solution B, the concentration of 2'deoxyguanosine was 7.4 g / L; Mix with solution B so that the ratio of BPDE to 2'deoxyguanosine is 1:50, mix well, keep away from light, and react at room temperature at 20°C for 50 hours.
[0060] 2. Initial purification
[0061] Weigh Si-C 18 The adsorbent is activated by methanol and loaded into the solid phase extraction column. The crude product solution is passed through the activated solid phase extraction column, and then rinsed with ultrapure water and methanol aqueous solution of different concentrations, and finally the column is eluted with methanol. Collect the eluent and concent...
Embodiment 3
[0066] 1. Synthesis of anti-BPDE-N 2 -dG
[0067] Weigh 0.05 mg (±) anti-BPDE and dissolve it in 100 μL of tetrahydrofuran: triethylamine = 23:1 solution to obtain A solution, the concentration of BPDE in A solution is: 0.5g / L; weigh 2.6 mg of 2'deoxyguanosine (dG) was dissolved in 1.2 mL 50 mmol / LTris-HCl pH=9 buffer solution to obtain solution B. In solution B, the concentration of 2'deoxyguanosine was 2.2 g / L, and solution A Mix with solution B so that the ratio of anti-BPDE to 2'deoxyguanosine is 1:60, mix well, keep away from light, and react at room temperature at 28°C for 40 hours.
[0068] 2. Initial purification
[0069] Weigh Si-C 18 The adsorbent is activated by methanol and loaded into the solid phase extraction column. The crude product solution is passed through the activated solid phase extraction column, and then rinsed with ultrapure water and methanol aqueous solution of different concentrations, and finally the column is eluted with methanol. Collect the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com