Method for recycling mixed acid of nitric acid, hydrofluoric acid and acetic acid
A technology of hydrofluoric acid and mixed acid, applied in the preparation of calcium/strontium/barium nitrate, carboxylate, calcium/strontium/barium fluoride, etc., can solve the problems of large amount of mixed acid, difficult filtration, and narrow application range. , to achieve the effect of low investment cost and operating cost, significant economic benefit and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
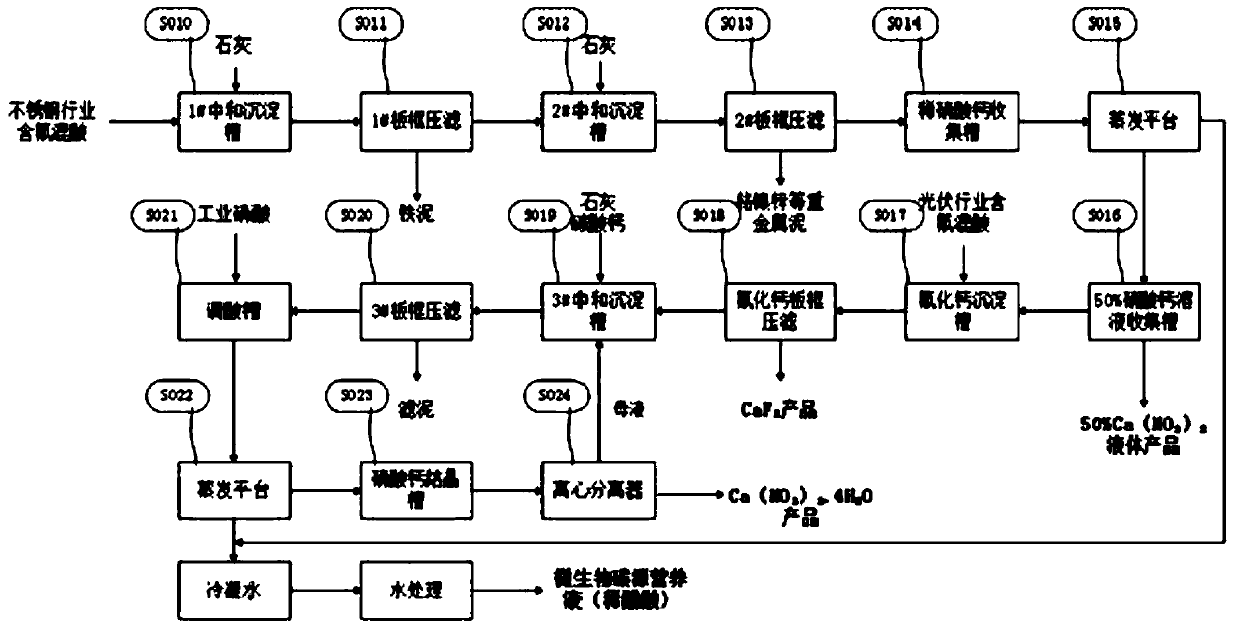
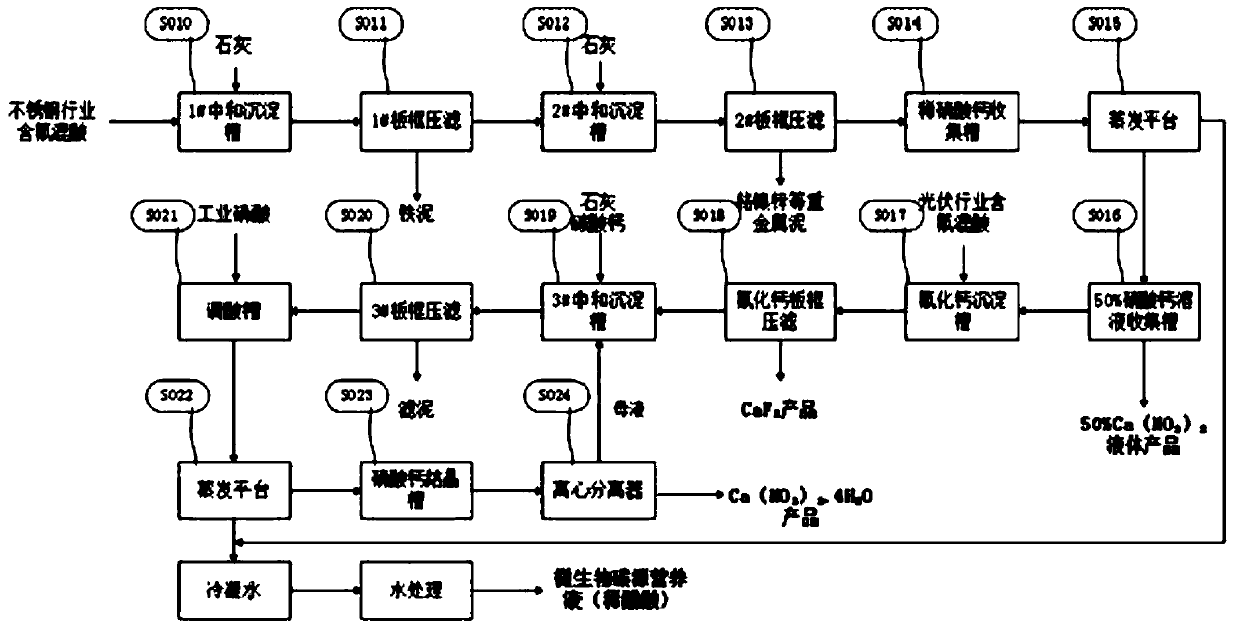
[0050] Adopt the inventive method to jointly process the fluorine-containing mixed acid that certain stainless steel enterprise and certain photovoltaic enterprise produce, wherein the mixed acid of stainless steel enterprise contains acidity (in terms of HNO 3 Total) mass fraction 2.94%, fluoride ion mass fraction 0.87%, iron ion mass fraction 2.61%, chromium ion mass fraction 0.34%; the mixed acid HNO of photovoltaic enterprises 3 The mass fraction is 15.12%, the HF mass fraction is 8.2%, and the HAc mass fraction is 7.87%. According to the inventive method, the processing steps are as follows:
[0051] S010, stainless steel fluorine-containing mixed acid in 1# neutralization sedimentation tank, adjust pH = 1 with lime, and mechanically stir to precipitate Fe 3+
[0052] After the reaction is completed, it is pumped to the 1# plate and frame filter press to separate the iron sludge through the filter press, and the filtered filtrate is sent to the 2# neutralization sedimen...
Embodiment 2
[0066] Adopt the inventive method to jointly process the fluorine-containing mixed acid that certain stainless steel enterprise and certain photovoltaic enterprise produce, the mixed acid of its stainless steel enterprise contains acidity (in terms of HNO 3 Total) 2.94%, fluoride ion 0.87%, iron ion 2.61%, chromium ion 0.34%; the mixed acid HNO of photovoltaic enterprises 3 The mass fraction is 15.12%, the HF mass fraction is 8.2%, and the HAc mass fraction is 7.87%. According to the inventive method, the processing steps are as follows:
[0067] S010, stainless steel fluorine-containing mixed acid in 1# neutralization sedimentation tank, adjust pH=2 with lime, mechanically stir to precipitate Fe 3+
[0068] After the reaction is completed, it is pumped to the 1# plate and frame filter press to separate the iron sludge through the filter press, and the filtered filtrate is sent to the 2# neutralization sedimentation tank.
[0069] S012, after the filtrate of 1# plate and fr...
Embodiment 3
[0082] Adopt the inventive method to jointly process the fluorine-containing mixed acid that certain stainless steel enterprise and certain photovoltaic enterprise produce, the mixed acid of its stainless steel enterprise contains acidity (in terms of HNO 3 Total) 2.94%, fluoride ion 0.87%, iron ion 2.61%, chromium ion 0.34%; the mixed acid HNO of photovoltaic enterprises 3 The mass fraction is 15.12%, the HF mass fraction is 8.2%, and the HAc mass fraction is 7.87%. According to the inventive method, the processing steps are as follows:
[0083] S010, stainless steel fluorine-containing mixed acid in 1# neutralization sedimentation tank, adjust pH=3 with lime, and mechanically stir to precipitate Fe 3+
[0084] After the reaction is completed, it is pumped to the 1# plate and frame filter press to separate the iron sludge through the filter press, and the filtered filtrate is sent to the 2# neutralization sedimentation tank.
[0085] S012, after the filtrate of 1# plate an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com