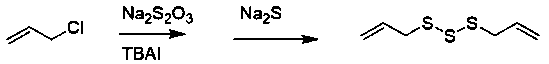Novel method for synthesizing allicin
A new method, the technology of allicin, is applied in the field of synthesis of diallyl trisulfide, the main component of allicin, and can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] The establishment of embodiment 1 synthetic route:
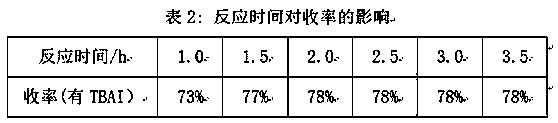
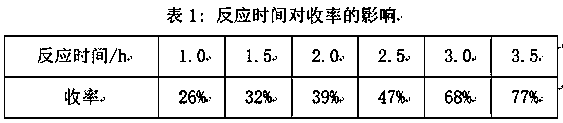
[0008] Add 36 g of sodium thiosulfate (containing 5 crystalline water) and 95 mL of distilled water into a 250 mL three-necked round-bottomed reaction flask with a magnetic stirring and reflux device, stir and heat up to about 35°C, then pipette 9.0 mL Allyl chloride was slowly dropped into the reaction flask in the dropping funnel, and the reaction temperature was controlled at 50±2°C to reflux. After reacting for 2 hours, cool to room temperature, and drop 10 mL of 1.8 mol / L sodium sulfide solution, stirred at room temperature for 30 min, the reaction solution was transferred to a separatory funnel, and stood overnight, discarded the lower water phase, washed the oil phase with distilled water for 3 times, and separated the water phase to obtain a light yellow oil, which was washed with anhydrous Na 2 SO 4 dry, filter out Na 2 SO 4 . Carry out vacuum distillation, collect fractions (59~60°C / 133.33 Pa), the cal...
Embodiment 2
[0010] The selection of embodiment 2 catalyst:
[0011] Add 36 g of sodium thiosulfate (containing 5 crystal water) and 95 mL of distilled water into a 250 mL three-necked round-bottomed reaction flask with a magnetic stirring and reflux device, stir and heat up to about 35 °C, add 0.05 g Phase-transfer catalyst tetra-n-butylammonium iodide, then pipette 9.0 mL of allyl chloride into the dropping funnel, slowly drop it into the reaction bottle, control the reaction temperature at 50±2°C and reflux, react for 2 h, cool After reaching room temperature, add 10 mL of 1.8 mol / L sodium sulfide solution dropwise into the reaction solution, stir at room temperature for 30 min, transfer the reaction solution into a separatory funnel, let it stand overnight, discard the lower aqueous phase, and wash the oil phase with distilled water 3 times, the water phase was separated to obtain a light yellow oil, which was washed with anhydrous Na 2 SO 4 dry, filter out Na 2 SO 4 , carry out va...
Embodiment 3
[0013] The selection of embodiment 3 catalyst concentration:
[0014] Add 36 g of sodium thiosulfate (containing 5 crystal water) and 95 mL of distilled water into a 250 mL three-necked round-bottomed reaction flask with a magnetic stirring and reflux device, stir and heat up to about 35 °C, add 0.1 g Phase-transfer catalyst tetra-n-butylammonium iodide, then pipette 9.0 mL of allyl chloride into the dropping funnel, slowly drop it into the reaction bottle, control the reaction temperature at 50±2°C and reflux, react for 2 h, cool After reaching room temperature, add 10 mL of 1.8 mol / L sodium sulfide solution dropwise into the reaction solution, stir at room temperature for 30 min, transfer the reaction solution into a separatory funnel, let it stand overnight, discard the lower aqueous phase, and wash the oil phase with distilled water 3 times, the water phase was separated to obtain a light yellow oil, which was washed with anhydrous Na 2 SO 4 dry, filter out Na 2 SO 4 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com