Amisulpride oral-administration solution
An amisulpride and oral solution technology, which can be applied in the directions of inactive components of polymer compounds, nervous system diseases, and pharmaceutical formulations, etc., can solve problems such as bad taste and increase drug solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
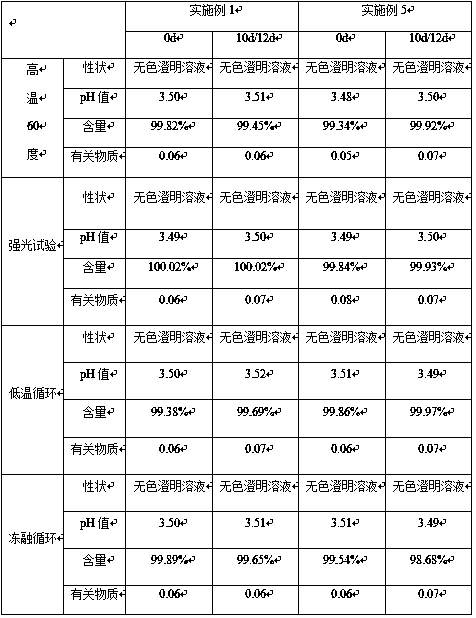
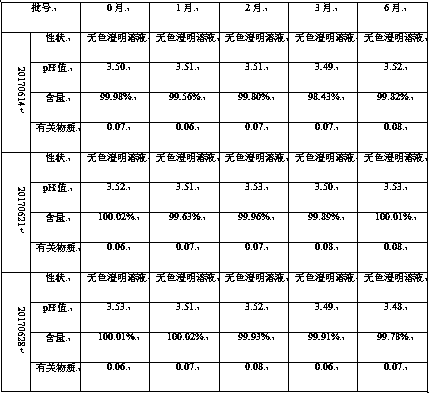
[0017] Prepare 1000mL of amisulpride concentration of 5% (w / v) oral solution, which contains the following components: amisulpride 50g, hydroxypropyl-β-cyclodextrin 150g, propylene glycol 300ml, 70% sorbitol 200ml , 0.5g Tween 80, 0.1g ethylparaben, 0.5g sodium saccharin, appropriate amount of sodium hydroxide, add distilled water to 1000ml.
[0018] Preparation method: first add 0.5g Tween 80 to 200ml of distilled water, add 150g of hydroxypropyl-β cyclodextrin and stir, continue to add 300ml of propylene glycol and stir evenly; add 50g of amisulpride into hydroxypropyl-β under constant stirring In the cyclodextrin solution, the inclusion complex solution of amisulpride was obtained, and then 200ml of 70% sorbitol solution, 0.1g of ethylparaben, 0.5g of sodium saccharin were added, and the pH was adjusted to 3.5 with 1mol / L sodium hydroxide solution. The solution obtained was diluted to 1000ml with distilled water, filtered to obtain amisulpride oral solution.
Embodiment 2
[0020] Prepare 1000mL of amisulpride concentration of 8% (w / v) oral solution, which contains the following components: amisulpride 80g, hydroxypropyl-β-cyclodextrin 240g, propylene glycol 200ml, 70% sorbitol 200ml , 0.5g Tween 80, 0.1g benzoic acid, 0.5g sodium saccharin, appropriate amount of sodium hydroxide, add distilled water to 1000ml.
[0021] Preparation method: first add 0.5g Tween 80 to 200ml distilled water, add 240g hydroxypropyl-β cyclodextrin and stir, continue to add 200ml propylene glycol and stir evenly; add 80g amisulpride to hydroxypropyl-β under constant stirring In the cyclodextrin solution, the clathrate solution of amisulpride was obtained, and then 200ml of 70% sorbitol solution, 0.1g of benzoic acid, 0.5g of sodium saccharin were added, and the pH was adjusted to 3.5 with 1mol / L sodium hydroxide solution to obtain the The solution was diluted to 1000ml with distilled water, filtered to obtain amisulpride oral solution.
Embodiment 3
[0023] Prepare 1000mL of amisulpride concentration of 2% (w / v) oral solution, which contains the following components: amisulpride 20g, hydroxypropyl-β-cyclodextrin 100g, propylene glycol 400ml, 70% sorbitol 200ml , 0.5g Tween 80, 0.1g benzoic acid, 0.5g sodium saccharin, appropriate amount of sodium hydroxide, add distilled water to 1000ml.
[0024] Preparation method: first add 0.5g Tween 80 to 200ml of distilled water, add 100g of hydroxypropyl-β cyclodextrin and stir, continue to add 400ml of propylene glycol and stir evenly; add 20g of amisulpride into hydroxypropyl-β under constant stirring In the cyclodextrin solution, the clathrate solution of amisulpride was obtained, and then 200ml of 70% sorbitol solution, 0.1g of benzoic acid, 0.5g of sodium saccharin were added, and the pH was adjusted to 3.5 with 1mol / L sodium hydroxide solution to obtain the The solution was diluted to 1000ml with distilled water, filtered to obtain amisulpride oral solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com