Mitochondrial aldehyde dehydrogenase-2 modulators for protecting, expanding and increasing potency of hematopoietic stem cells
A technology of hematopoietic stem cells and hematopoietic cells is applied in the field of in vitro expansion of hematopoietic cells and improvement of hematopoietic cell efficacy, protection, expansion of hematopoietic cells and improvement of hematopoietic cell efficacy, and can solve the problem of less and sufficient expansion of HSC. Issues such as increasing HSC and HSC transplantation application restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0230] The following examples are presented to provide one of ordinary skill in the art with a complete disclosure and description of how to make and use the invention, and are not intended to limit the scope of what the inventors believe to be their invention, nor to represent that the following experiments were all or The only experiment. Efforts have been made to ensure accuracy with respect to numbers used (eg, amounts, temperature, etc.), but some experimental errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, molecular weight is weight average molecular weight, temperature is in degrees Centigrade, and pressure is at or near atmospheric. Standard abbreviations can be used, for example, bp, base pair; kb, kilobase; pl, picoliter; s or sec, second; min, minute; h or hr, hour; aa, amino acid; kb, kilobase; bp, base pair; nt, nucleotide; i.m., intramuscular (ground); i.p., intraperitoneal (ground); s.c., subcutaneous (groun...
example 1
[0231] Example 1: Effect of increased aldehyde load on HSC interferon stress response.
[0232] DNA interstrand crosslinks (ICLs), normally repaired by the Fanconi anemia (FA) complex gene products, can be induced by reactive aldehydes (eg, acetaldehyde). A hypomorphic missense mutation of aldehyde dehydrogenase 2 (ALDH2) is found in about 560 million people worldwide, resulting in a reduced ability to metabolize acetaldehyde and other toxic aldehydes. The ALDH2*2 genotype causes the well-known disulfiram-like "Asian flushing syndrome" observed after ethanol intake, but also increases susceptibility to cancer, aplastic anemia, Higher risk and faster progression to bone marrow failure.
[0233] The effect of increased aldehyde load in HSCs caused by genetic variation (ALDH2*2) and environmental exposure (ethanol treatment (challenge) to increase aldehyde load) was investigated. Hematopoietic stem progenitor cells (HSPCs) from wild-type (WT) and ALDH2*2 / *2 mice between 6-30 we...
example 2
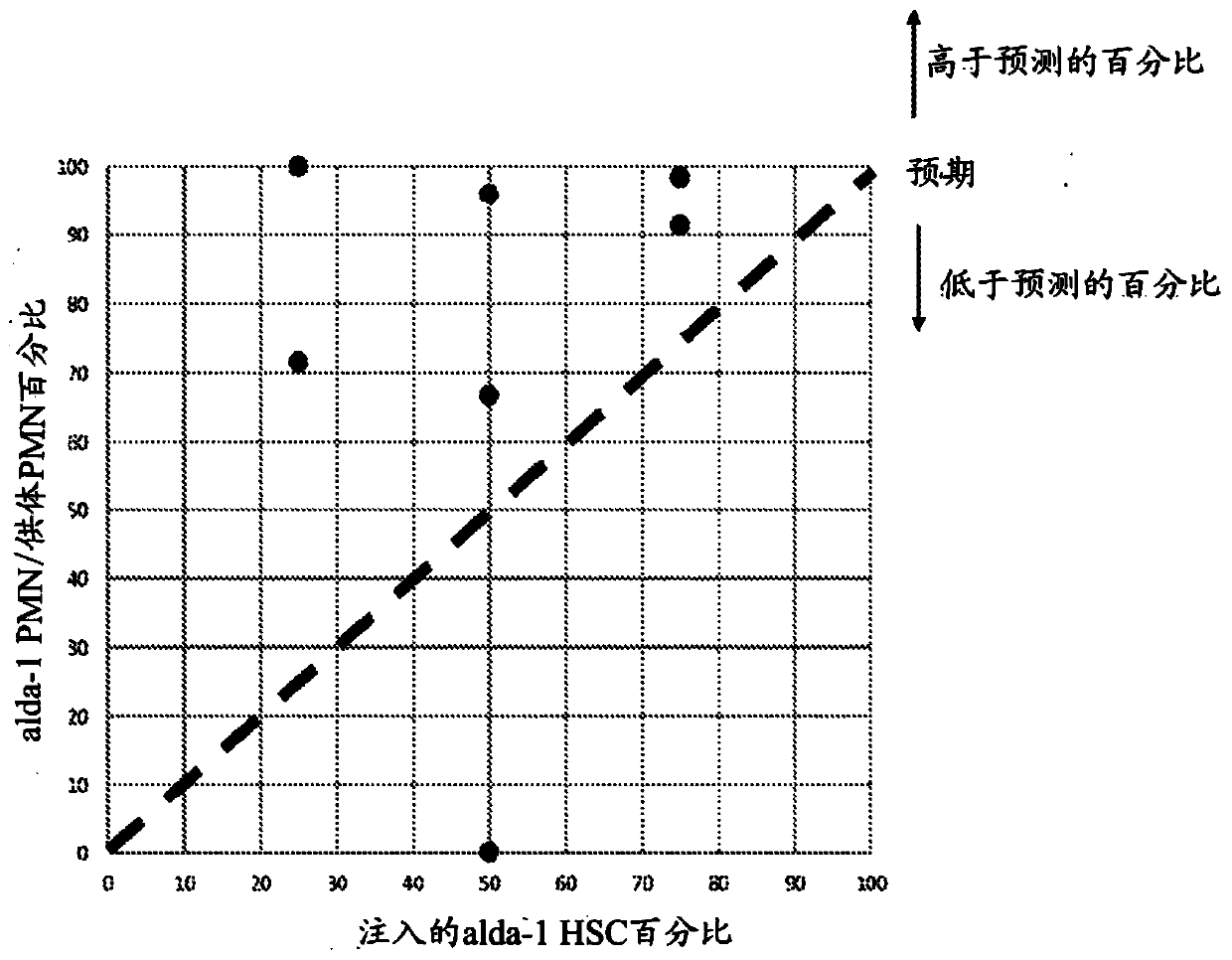
[0237] Example 2: ALDH2 activators protect and expand HSCs in vivo.
[0238] Mice (wild-type C57BL / 6N) were administered Alda-1 (10 mg / kg.day) or vehicle control (50% DMSO / 50% PEG-400) for 3 months by continuous infusion via a subcutaneous osmotic pump.
[0239]
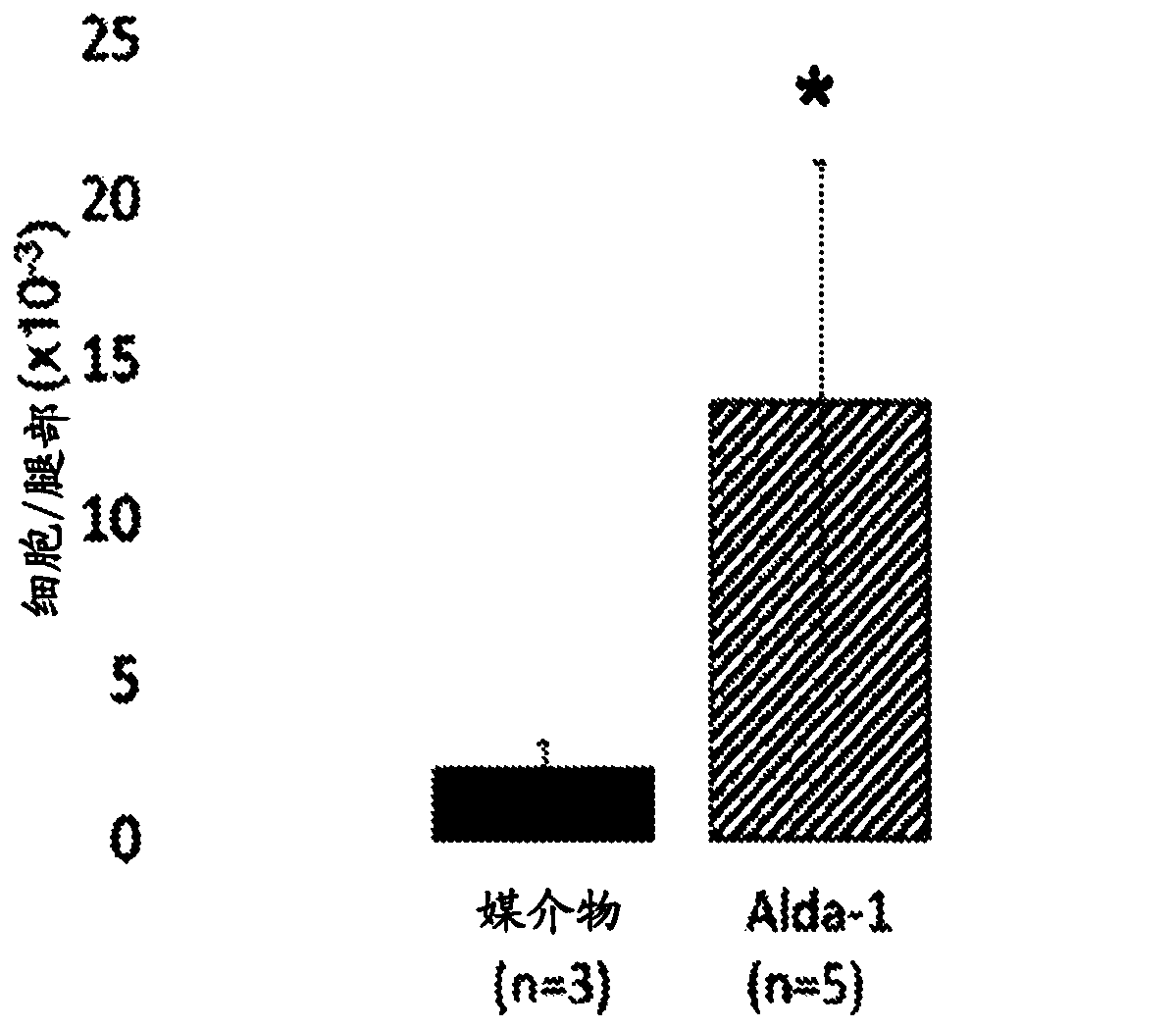
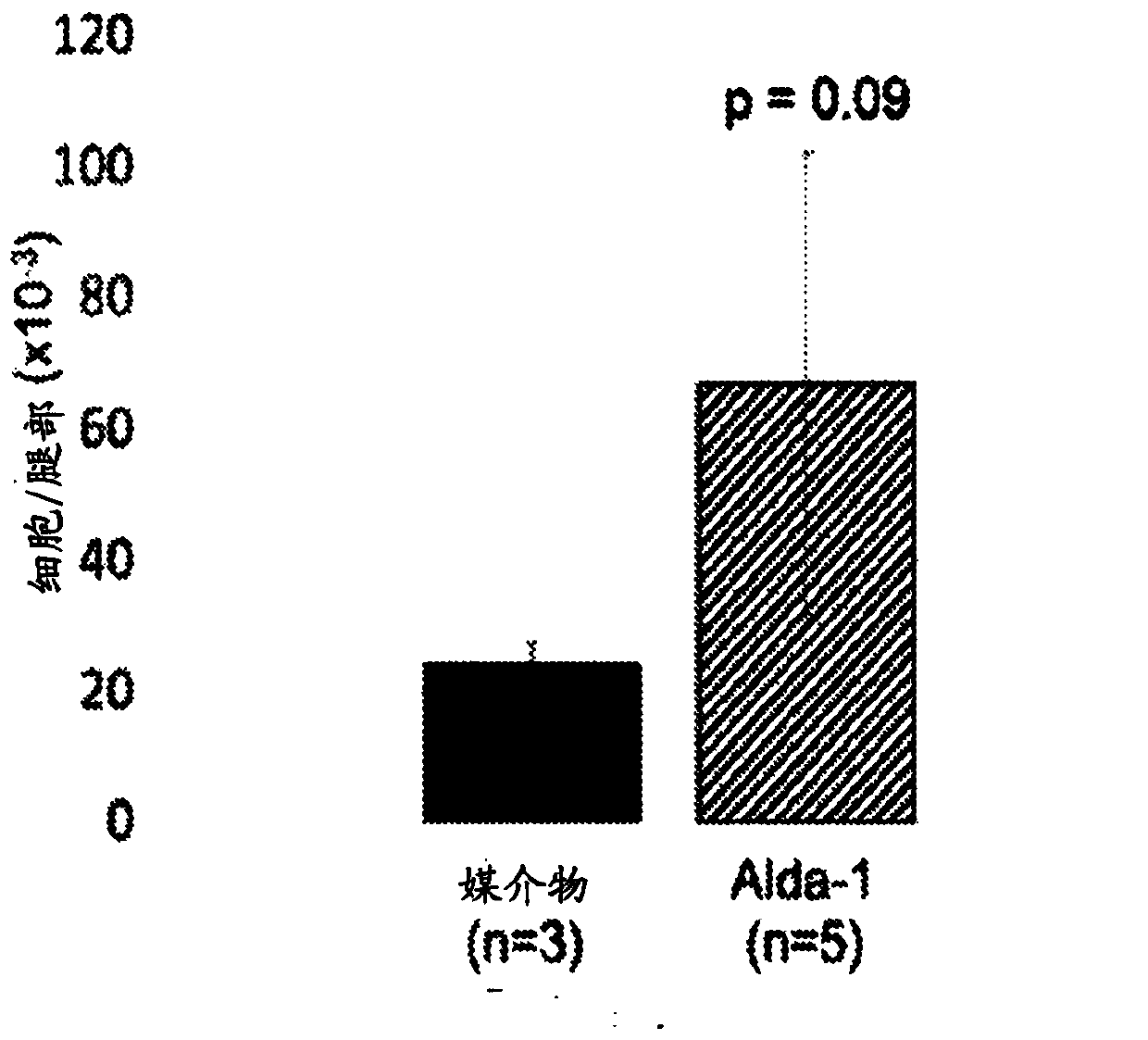
[0240] HSC function was then analyzed in vehicle control and Alda-1 treated mice. Mice treated with Alda-1 exhibited marked HSC expansion. It was observed that mice treated with Alda-1 exhibited a 4-fold increase in (LT)-HSC ( Figure 1A ), and mice treated with Alda-1 exhibited a 2-fold increase in (ST)-HSC ( Figure 1B ). see Figure 1A and Figure 1B , error bars show standard deviation, significance calculated with Student's t-test. *=p≤0.05. In conclusion, chronic Alda-1 administration was well tolerated in vivo and resulted in the expansion of HSCs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com