A compound capable of increasing skin anti-wrinkle function and its use in preparing cosmetics
A technology of anti-wrinkle cosmetics and compounds, applied in the field of biomedicine, can solve the problems that the improvement effect needs to be further improved, and achieve good market prospects, low cytotoxicity, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of coupling compound
[0023] Preparation of polypeptide-compound conjugate The compound of formula (II) of the present invention was synthesized according to the compound synthesis route in step A3 of Example A3 in CN201480017060.2. Then add 1mmol formula (II) compound, 1mmol O-benzotriazole-N,N,N',N'-tetramethylpirouentetrafluoroborate (TBTU), 5mlDMF, nitrogen protection in 50m reaction bottle, Add 0.5mmol) N, N-diisopropylhexylamine (DIEA) dropwise, stir at 25°C for 1.5h, add 0.1g of cell activity-promoting peptide (sequence VNHNYDFPWPDSEGKPPPHWLAKSPQK), stir at 36°C for 3.5h, and stop reaction. After confirming the correct molecular weight using MS-IT-TOF, the crude product was purified using HPLC to obtain a purified product with a purity of 99.96%. The structural formula of the conjugated product obtained through structural analysis is shown in formula (I): .
Embodiment 2
[0024] Example 2 Compound Toxicity Detection
[0025] (1) Cell culture
[0026] At 37°C, 5% CO 2 Human fibroblasts were prepared by culturing in DMEM medium containing 100 U / ml penicillin / streptomycin and 10% FBS in an incubator.
[0027] (2) Preparation of medium containing the compound of formula (I)
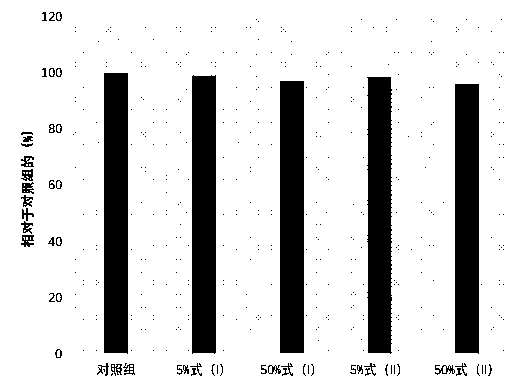
[0028] Mix DMEM powder (GIBCO, New York, USA) in the compound of Example 1, then add antibiotics (penicillin / streptomycin of 100U / ml) and 10% FBS to prepare the medium containing 5% formula (I) compound As well as the culture medium containing 50% of the compound of formula (I), the culture medium without the compound was used as a blank control, and the culture medium added with the compound of formula (II) was used as a positive control, and the concentrations were also 5% and 50% respectively.
[0029] (3) Cytotoxicity test of compounds
[0030] The human fibroblasts prepared in step (1) were added to a 24-well plate and cultured for 24 hours, and then the medium was re...
Embodiment 3
[0032] Example 3 Detection of COL1 protein expression by Western blotting electrophoresis
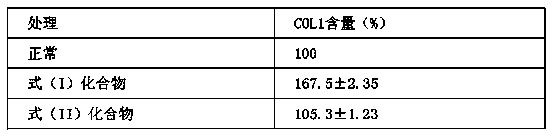
[0033]Human skin fibroblasts (HFF-1) were cultured in DMEM medium containing 15% fetal calf serum to the logarithmic phase of growth, digested with 0.25% trypsin, and seeded in 6-well plates at 105 / ml, each well 2ml. Cultivate the cells in a CO2 incubator at 37°C overnight to make the cells adhere to the wall. When the cell density grows to about 80%, the medium is aspirated, and the phosphate buffer solution (PBS: 0.8gNaCl; 0.2g KCl; 2.9g Na2HPO4.12H2O ; Dissolve 0.27g KH2PO4 in 800ml deionized water, stir to dissolve, dilute to 1L, adjust pH to 7.4 with concentrated HCl, autoclave) wash cells 2-3 times. Add the compound sample with a final concentration of 5% formula (I), add the compound sample with a final concentration of 5% formula (II) as a control, add 2% serum DMEM to the blank and incubate for 24 hours. Then discard the cell supernatant, centrifuge at 1000 g for 5 min to col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com