Chlorate decomposition process method in ionic membrane caustic soda production
A technology of ionic membrane method and process method, which is applied in the field of chlorate decomposition process in the production of ionic membrane method caustic soda, which can solve the problem that the decomposition flow rate of chlorate decomposition device can no longer be increased, affect the economic benefits of chlor-alkali system, and affect the quality of liquid caustic soda products and other problems, to achieve the effect of saving auxiliary material consumption, reducing the number of inspection and maintenance, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] In order to describe the technical content, achieved objectives and effects of the present invention in detail, the following descriptions will be made in conjunction with the embodiments.
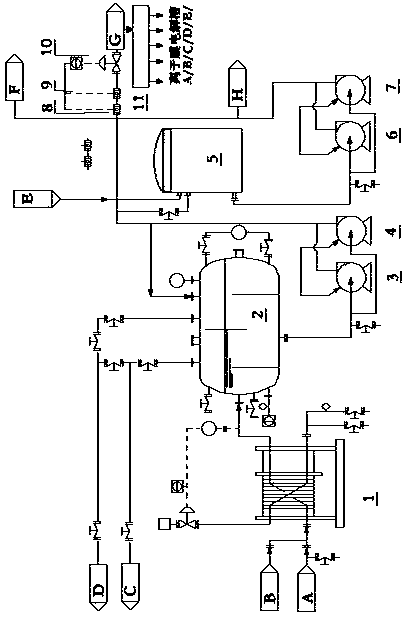
[0045] Refer to attached figure 1 , embodiment 1 of the present invention in order to achieve the above object, proposes a kind of chlorate decomposition process in the production of caustic soda by ion-exchange membrane method, its purpose aims at overcoming the defective of prior art, realizes chlorate decomposition and subsequent production process , including the following steps:
[0046] Step 1, send the light brine through the chlorate heat exchanger 1 to the outlet of the chlorate decomposition tank 2 by the chlorate pump A or B3 or 4 to the electrolytic anode circulation tank 5;
[0047] Step 2, the above-mentioned outlet light brine can also be directly sent to the light brine pipeline for protecting the refined brine main pipe by the chlorate pump 3 or 4, and the saturate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com