Adsorbent resin and synthetic method thereof and method for treating wastewater
A technology for adsorbing resin and wastewater, applied in the fields of adsorbing water/sewage treatment, water/sewage treatment, chemical instruments and methods, etc., can solve the problems of weak intermolecular force, small adsorption capacity, poor adsorption effect, etc., to overcome the removal of Incomplete, stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
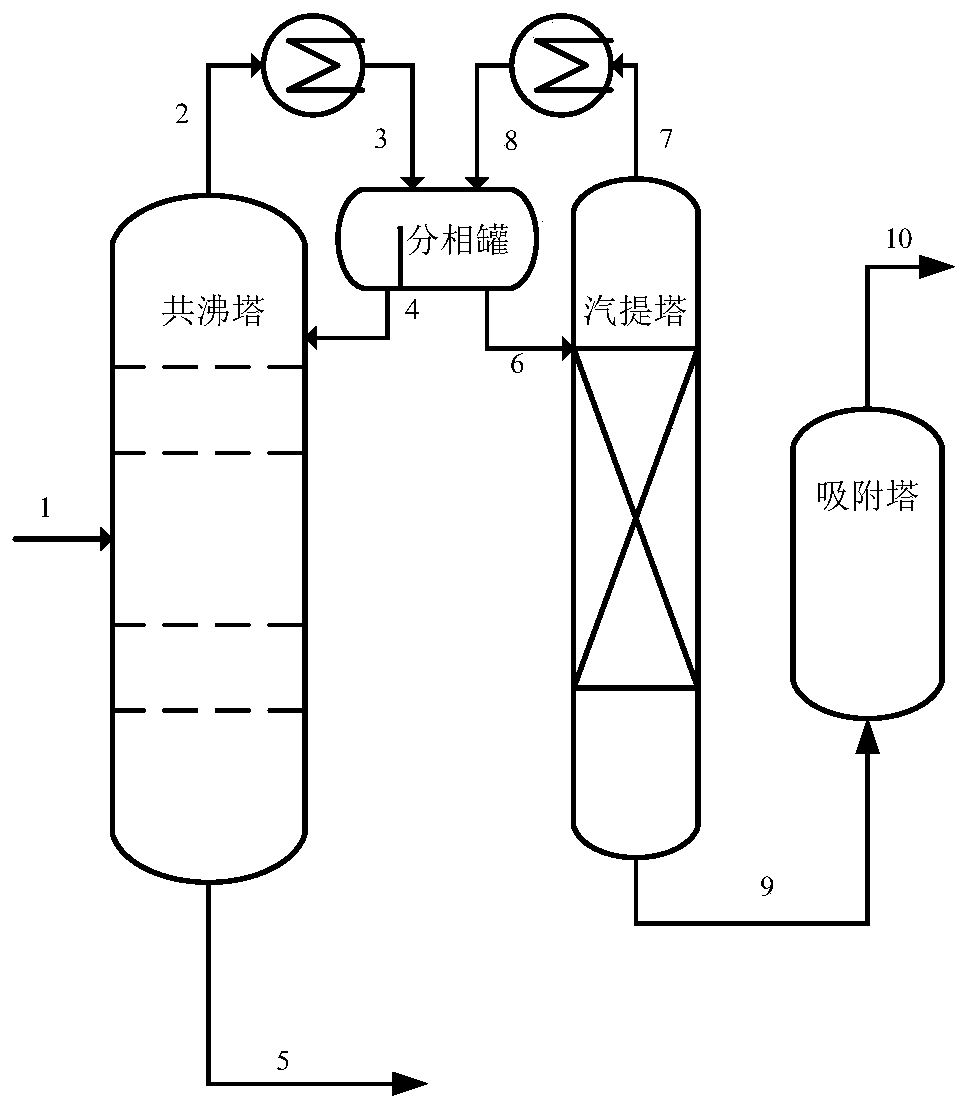
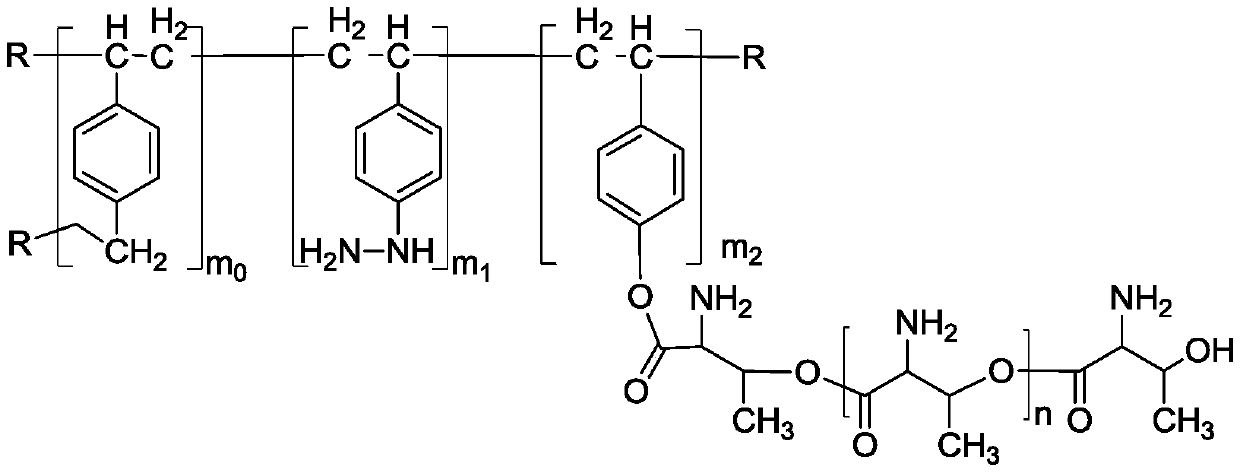
Image
Examples
Embodiment 1
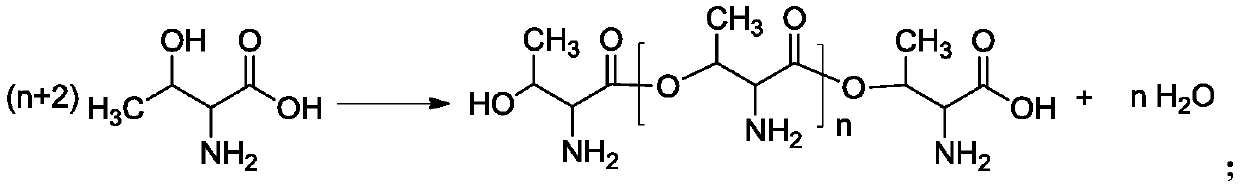
[0083] Add α-amino-β-hydroxybutyric acid 88.0g and 0.0.53g methanesulfonic acid (the ratio is 0.6wt%) to the reactor equipped with stirring, thermometer, reflux condenser and N,N-dimethylformamide solution , heated to 60°C at a stirring speed of 200 rpm and an absolute pressure of 35kPa, and reacted for 1.5h. After the reaction was completed, 8.5 g of p-hydroxystyrene and 0.2 g of polymerization inhibitor 701 were added; the temperature was raised to 120° C. to continue the reaction for 2.0 h. After the reaction was completed, 9.7 g of deionized water was added to extract methanesulfonic acid to obtain hydroxystyrene with pendant amino groups.
[0084] The molecular weight distribution of hydroxystyrene with side amino groups measured by GPC is as follows, and the range of n corresponding to the molecular weight distribution is 2 to 19:
[0085] molecular weight 500-1500 1500—2500 content 32% 68%
[0086] 56.8 g of p-chlorostyrene and 25.6 g of hydrazi...
Embodiment 2
[0091] Add α-amino-β-hydroxybutyric acid 61.5g and 0.5g methanesulfonic acid (ratio is 0.8wt%) in the reactor equipped with stirring, thermometer, reflux condenser, N,N-dimethylformamide solution, Heat to 80°C at a stirring speed of 250 rpm and an absolute pressure of 25kPa, and react for 2.0h. After the reaction was completed, 5.3 g of p-hydroxystyrene and 0.15 g of polymerization inhibitor 701 were added; the temperature was raised to 100° C. to continue the reaction for 1.5 h. After the reaction was completed, 6.7 g of deionized water was added to extract methanesulfonic acid to obtain hydroxystyrene with pendant amino groups.
[0092] The molecular weight distribution of hydroxystyrene with side amino groups measured by GPC is as follows, and the range of n corresponding to the molecular weight distribution is 2 to 19:
[0093] molecular weight 500-1500 1500—2500 content 26% 74%
[0094] Add 77.3 g of p-chlorostyrene and 15.8 g of hydrazine hydrate...
Embodiment 3
[0099] Add α-amino-β-hydroxybutyric acid 57.3g and 0.6g (1.1wt%) methanesulfonic acid in the reactor equipped with stirring, thermometer, reflux condenser, N,N-dimethylformamide solution, at 250 Stirring speed at rev / min, heating to 70°C under 25kPa absolute pressure, and reacting for 1.5h. After the reaction was completed, 4.2 g of p-hydroxystyrene and 0.15 g of polymerization inhibitor 701 were added; the temperature was raised to 130° C. to continue the reaction for 2.5 h. After the reaction was completed, 8.0 g of deionized water was added to extract methanesulfonic acid to obtain hydroxystyrene with pendant amino groups.
[0100] The molecular weight distribution of hydroxystyrene with side amino groups measured by GPC is as follows, and the range of n corresponding to the molecular weight distribution is 2 to 19:
[0101] molecular weight 500-1500 1500—2500 content 30% 70%
[0102] 19.3 g of p-chlorostyrene and 15.8 g of hydrazine hydrate were re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com