A kind of non-peripheral quaternary ammonium group modified zinc phthalocyanine and its preparation method and application
A technology of zinc phthalocyanine and quaternary ammonium group, which is applied in the field of non-peripheral quaternary ammonium group modified zinc phthalocyanine and its preparation. It can solve the problems of in-depth research on the structural characteristics of photosensitizers, lack of joint photosensitizers, and clinical application limitations, etc., and achieve good photodynamics. Anticancer activity, structural elucidation, efficacy in orthotopic tumor clearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
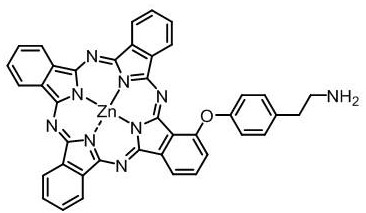
[0029] Non-peripheral quaternary ammonium group-modified zinc phthalocyanine (1-[4-(N,N,N-trimethyl-2-aminoethyl)phenoxy]zinc phthalocyanine iodide), the structure is shown in the following formula:
[0030]
[0031] Weigh 20 mg (28.5 μmol) of 1-[4-(aminoethyl)phenoxy]zinc phthalocyanine and K 2 CO 3 (168.28 μmol) was dissolved in a single-neck round-bottomed flask containing 10 ml of anhydrous DMF by sonication, cooled to 0 °C and slowly added with 2000 mg CH 3 1. After stirring for 30 min, the reaction was carried out at room temperature. TLC spot plate, stop the reaction after 24 h, spin dry the reaction solvent, dissolve the reactant with 5 ml DMF and filter with a 0.22 μm syringe filter to remove insoluble matter. The solvent was spun dry in vacuo, dissolved in 1 ml of DMF, and passed through an S-X1 gel column with DMF as the eluent, and the blue-green components at the forefront were collected. Vacuum and spin dry the solvent, dissolve it with EA and pass through ...
Embodiment 2
[0034] The target product can also be obtained by replacing the reaction solvent of Example 1 with 6 ml or 60 ml of anhydrous DMF, and other conditions remain unchanged. The structural characterization data of the product are as follows: 1 H NMR (400 MHz, DMSO) δ 9.23 (d, J = 23.3 Hz, 6H), 8.83 (s, 1H), 8.16 (s, 6H), 7.77 (d, J = 6.2 Hz, 1H), 7.44 (s, 2H), 7.37 (s, 2H), 7.09 (s, 1H), 3.90 (s, 2H), 2.74 (s, 2H), 1.50 (s, 2H), 1.26 (s, 4H),0.84 (s, 3H). HRMS (ESI) m / z calcd for C 43 H 32 N 9 OZn [M-I] + : 754.2016; found: 754.2042. HPLC (674 nm): > 95%..
Embodiment 3
[0036] 2000mg CH of Example 1 3 I, replaced with 1000mg CH 3 I or 4000mg CH 3 I, other conditions remain unchanged, the target product can also be obtained. The structural characterization data of the product are as follows: 1 H NMR (400 MHz, DMSO) δ 9.23 (d, J =23.3 Hz, 6H), 8.83 (s, 1H), 8.16 (s, 6H), 7.77 (d, J = 6.2 Hz, 1H), 7.44 (s, 2H), 7.37 (s, 2H), 7.09 (s, 1H), 3.90 (s, 2H), 2.74 (s, 2H), 1.50 (s, 2H), 1.26 (s, 4H), 0.84 (s, 3H). HRMS (ESI) m / z calcd for C 43 H 32 N 9 OZn [M-I] + : 754.2016; found: 754.2042. HPLC (674 nm): > 95%..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com