Recrystallization method of mirabegron and preparation method thereof
A recrystallization and crystallization technology, which is applied in the recrystallization method and preparation field of Mirabegron, can solve the problems of high raw material cost and cumbersome process steps, and achieve the effect of low production cost and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
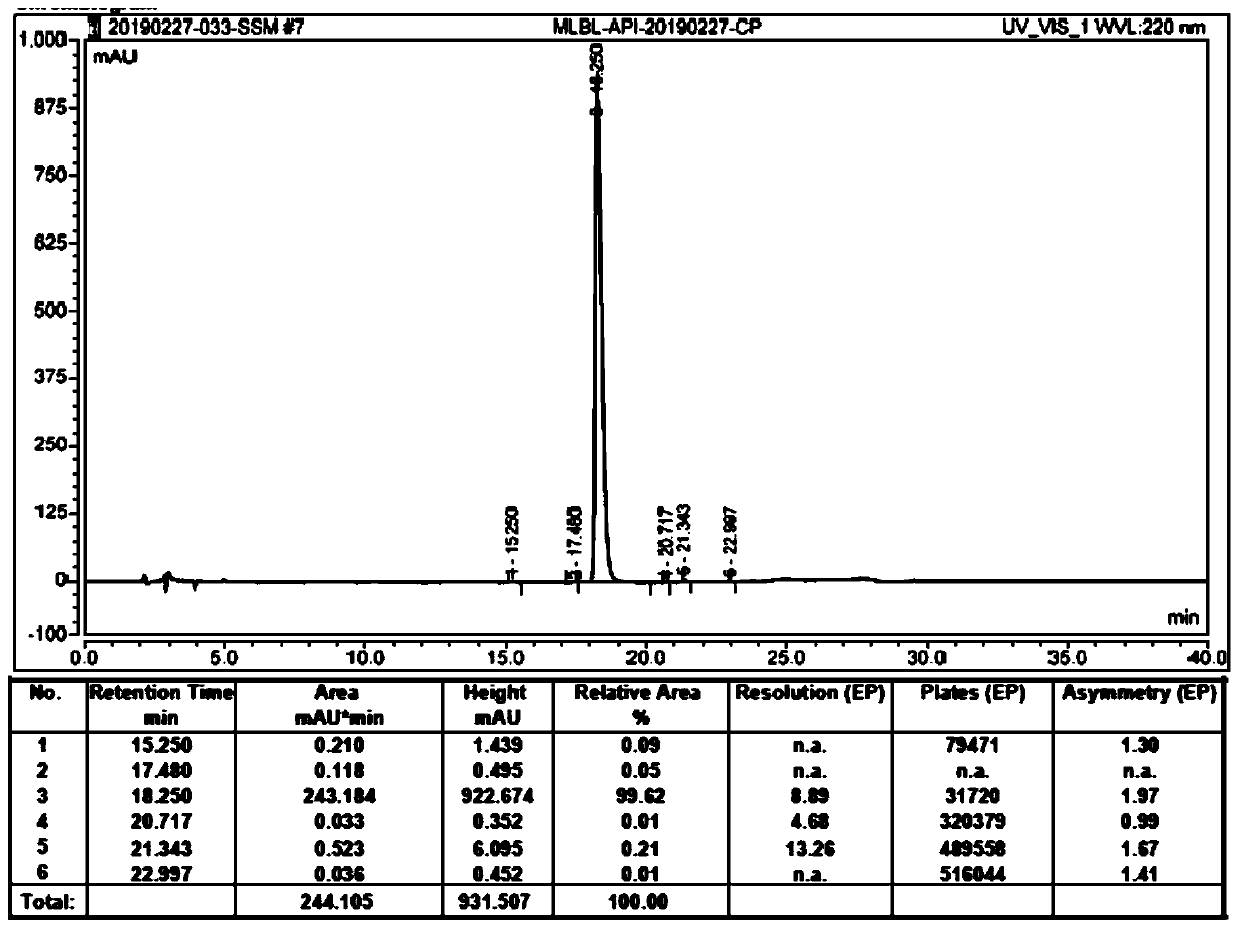
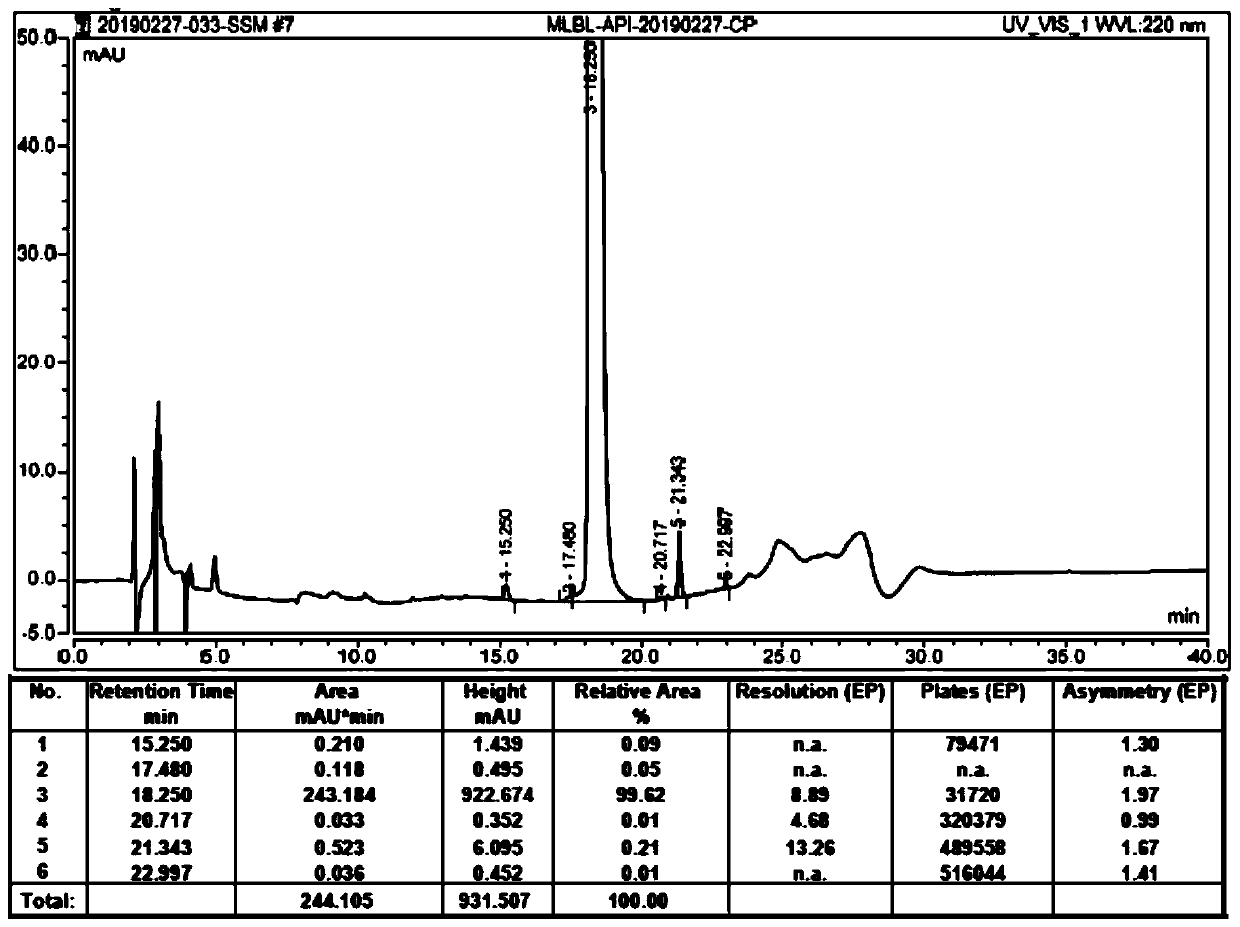
[0071] Add 400mL of water and 6.15g of concentrated hydrochloric acid to a 1L three-necked flask. After stirring, add 100g of (R)-2-(4-aminophenethylamino)-1-phenylethanol dihydrochloride, 48g The 2-aminothiazole-4-acetic acid and 70g EDCI were stirred at room temperature for 1h. HPLC monitoring, the reaction is complete. Transfer to a 2L beaker, add 400 mL of water, and slowly add 20% NaOH solution dropwise after stirring. A white solid precipitates. Adjust the pH to 9-10. After stirring for 30 minutes, filter. The filter cake was slurried with 2L of ethanol aqueous solution with a volume fraction of 15%, filtered with suction, and dried at 45-50° C. to obtain 114.2 g of crude Mirabegron with a yield of 94.8%, purity: 99.62%, and imA content: 0.21%. The liquid phase analysis spectrum of the crude Mirabegron is as follows figure 1 As shown, figure 2 for figure 1 An enlarged view of the liquid phase analysis spectrum.
[0072] The embodiment provides a recrystallization method ...
Embodiment 5-8
[0079] The embodiment provides a recrystallization method of Mirabegron, which includes the following steps:
[0080] Add the mixed solvent to the three-necked flask, heat the mixed solvent to the reflux temperature, add the crude Mirabegron under mechanical stirring at 150rpm, stir to dissolve, white solid precipitates, naturally cool to room temperature and stir for 3h, then filter, collect the solid , Dry the solid at room temperature by blowing air to obtain pure Mirabegron.
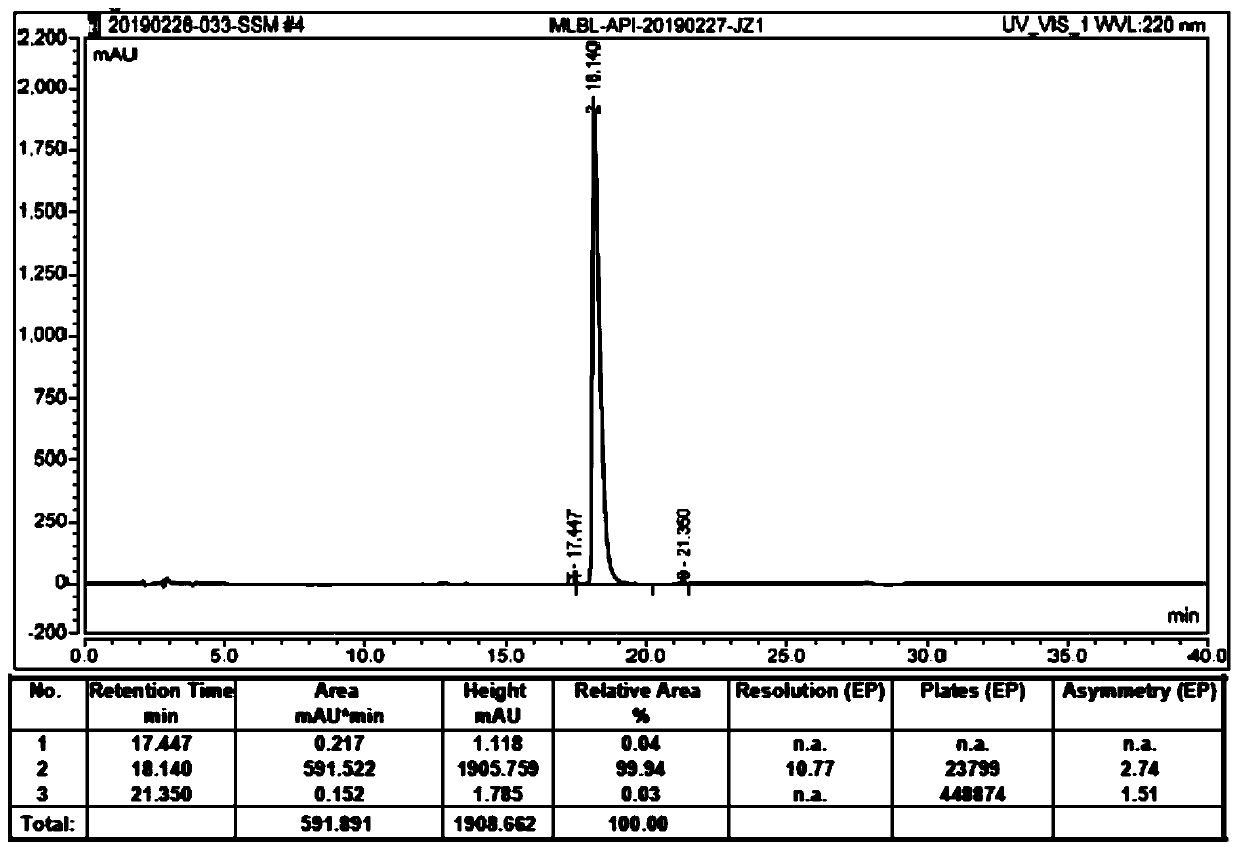
[0081] Among them, in the recrystallization process of different embodiments, the crude species and amount used, the composition and amount of the mixed solvent, the amount of pure Mirabegron obtained, the yield of pure Mirabegron, the purity of pure Mirabegron, The content of imA impurity in Mirabegron pure product is shown in Table 3-4.
[0082] Table 3 Operating conditions of different embodiments
[0083]
[0084] Table 4 Results of pure Mirabegron obtained by recrystallization in different examples
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com