l-arabinose isomerase, mutants and applications thereof
An arabinose and mutant technology, applied in the field of L-arabinose isomerase and its mutants, can solve the problems of restricting the production level of D-tagatose, unfavorable industrial production, low substrate concentration, etc., and achieve ultra-high substrate Conversion rate, improvement of industrialization level, effect of ultra-high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the screening of novel enzyme
[0022] 1. Source of enzyme and construction of recombinant bacteria
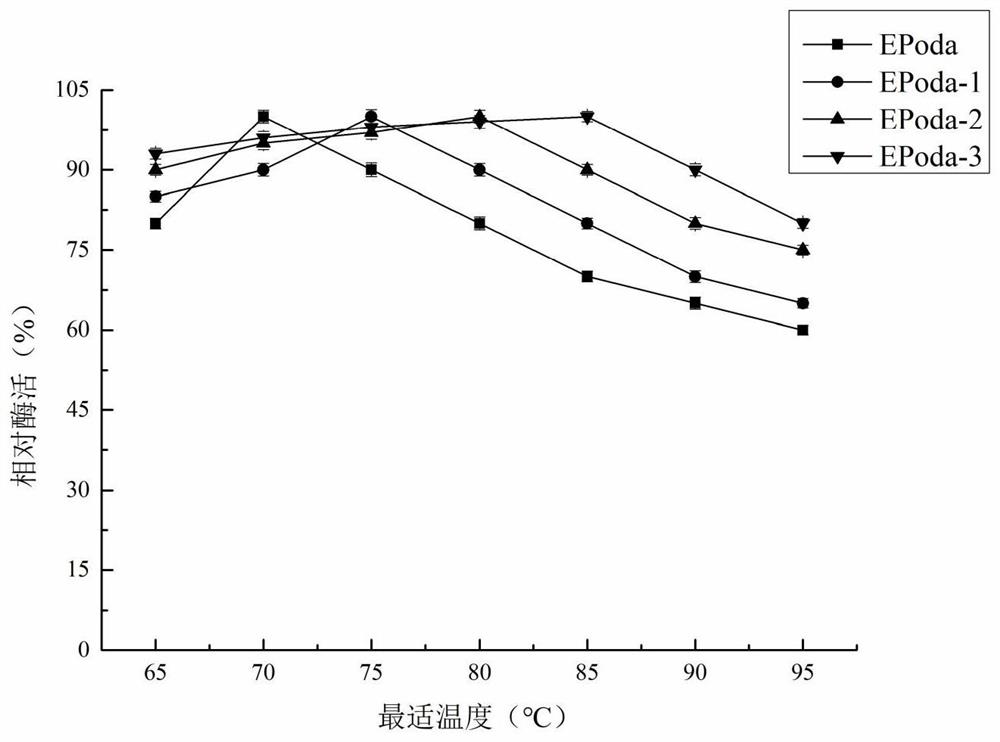
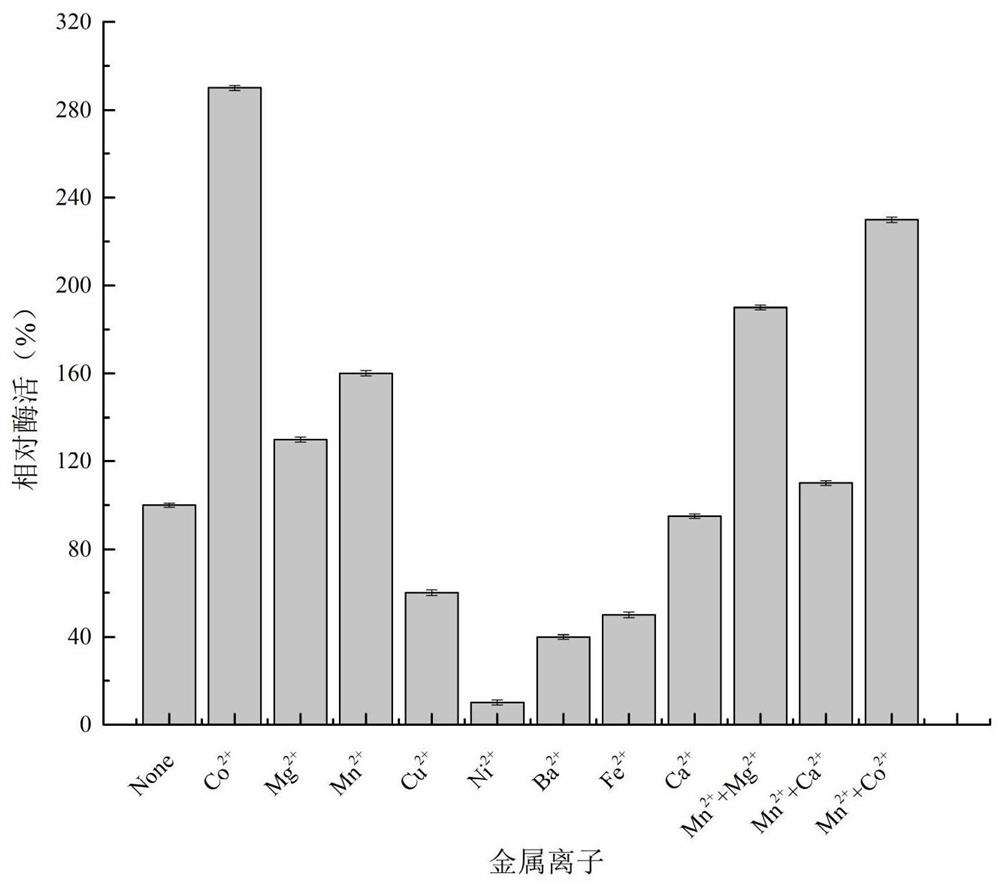
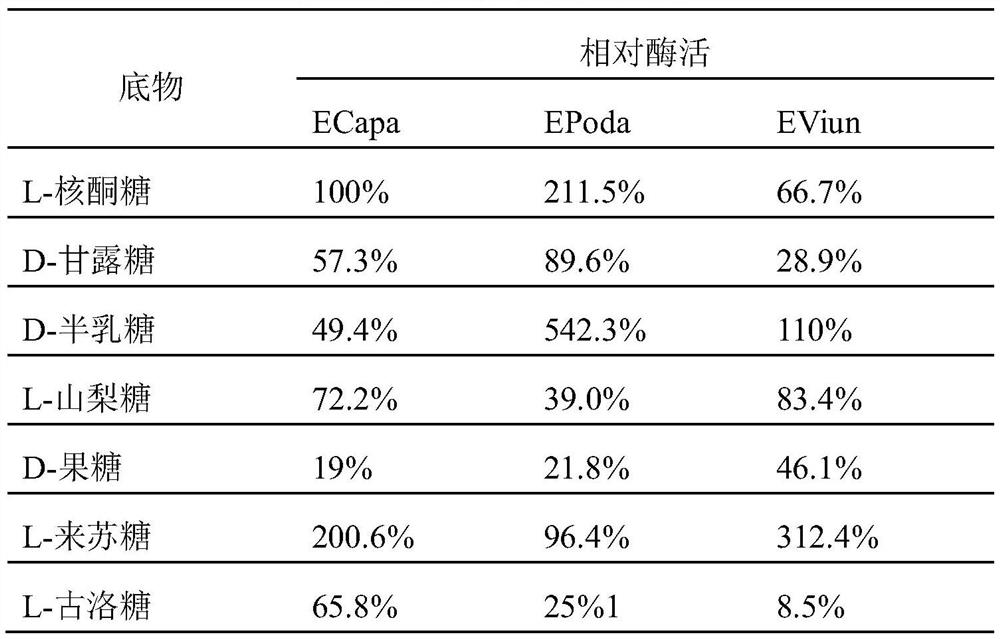
[0023] The key catalytic regions of isomerases were analyzed, and three unstudied sugar isomerases were obtained from the NCBI database, which were derived from Pocillopora damicornis (GenBank No. XP_027045535.1), Vignaunguiculata (GenBank No. Candidatus Paracaedibacteracanthamoebae (GenBank accession number WP_075261590.1), and named EPoda, EViun and ECapa. According to the amino acid sequence, the codon was optimized according to the codon preference of Escherichia coli, and three selected nucleotide sequences were synthesized by the method of total synthesis through the conventional operation of genetic engineering, such as SEQ ID NO.2, SEQ ID NO.4 and shown in SEQ ID NO.6; the amino acid sequences encoding the enzyme are shown in SEQ ID NO.1, SEQ ID NO.3 and SEQ ID NO.5 respectively. Add 6×His-tag tags at the end of the nucleic acid sequence, add res...
Embodiment 2
[0037] Example 2: Construction and screening of EPoda single point mutants
[0038] 1. Mutant construction
[0039] According to the parental sequence of EPoda (the amino acid sequence is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.2), the mutation primers for site-directed mutation were designed, and the recombinant vector pET28b / EPoda was used as a template by using rapid PCR technology. To introduce a single mutation at position 107, the primers are:
[0040] Forward primer 107I: AACTGGATGTGGCG NNN GATGGCGCGGATGATGTG (the underline is the mutated base)
[0041] Reverse primer 107I: CACATCATCCGCGCCATC NNN CGCCACATCCAGTT (the underline is the mutated base)
[0042] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ ) 25 μL, dNTPs 10 mM, forward primer 107I 2 μL (5 pmol / μL, the same below), reverse primer 107I 2 μL (5 pmol / μL, the same below), template DNA 1 μL (20ng / μL, the same below), Phanta Max Super- Fidelity DNA Polymerase 50U ...
Embodiment 3
[0054] Example 3: Construction and screening of EPoda double-site mutants
[0055] According to the single mutant EPoda-1 sequence constructed in Example 2, the mutation primers for site-directed mutation were designed, and the rapid PCR technology was used to use the recombinant vector pET28b / EPoda-1 as a template to introduce a single mutation at position 199. The primers were:
[0056] Forward primer 199D: GGCCCGGTGGTGACC NNN AACAGCAACTTTATTA (the underline is the mutated base)
[0057] Reverse primer 199D: TAATAAAGTTGCTGTT NNN GGTCACCACCGGGCC (the underline is the mutated base)
[0058] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ ) 25μL, dNTPs 10mM, forward primer 199D 2μL, reverse primer 199D 2μL, template DNA 1μL, Phanta Max Super-Fidelity DNA Polymerase 50U, add ddH 2 0 to 50 μL.
[0059] The PCR amplification conditions were 95°C for 3min; (95°C for 15s, 63°C for 15s, 72°C for 6.5min) for 30 cycles; 72°C for 5min.
[0060] The PCR product was tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com