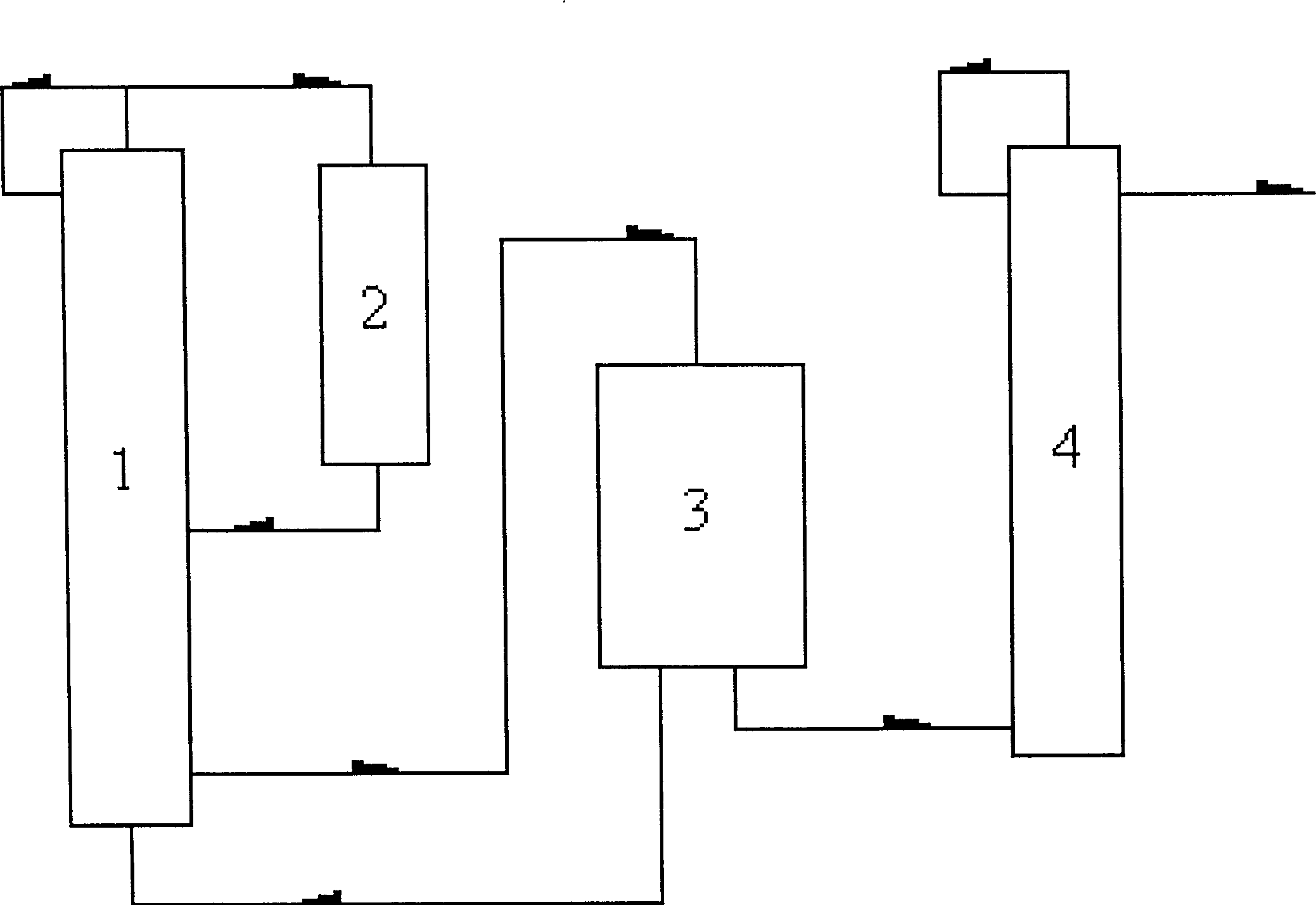
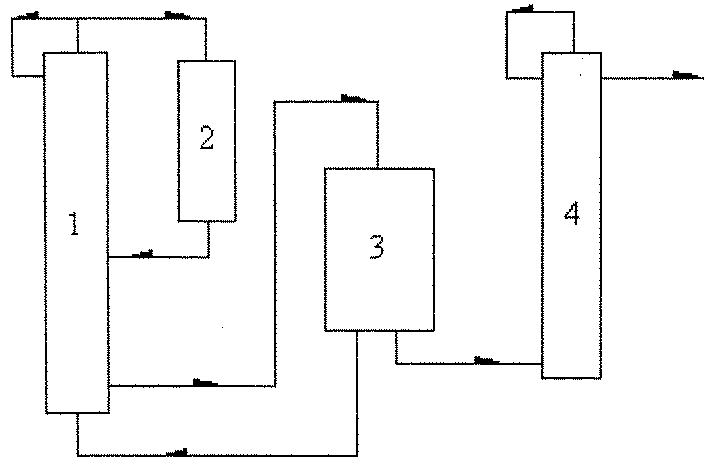
Process for synthesizing 2-chloropyridine from chlorine and pyridine
A technology for chloropyridine and pyridine, which is applied in the field of improvement of the synthetic 2-chloropyridine process, can solve the problems of difficult separation, difficult temperature control, large waste liquid discharge and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Pyridine: Origin: Tianjin Second Chemical Reagent Factory, purity ≥ 99%
[0014] Chlorine: Origin: Tianjin Dagu Chemical Plant, purity ≥ 99%
[0015] Using the above-mentioned equipment and process flow, mix 500g of pyridine and 340ml of water, feed 428g of chlorine gas, the reaction temperature is 142-155°C, the reaction time is 180 minutes, and 142g of NaOH is used for neutralization to obtain 640.3g of 2-chloropyridine, unreacted pyridine 24.3 g, the selectivity is 94.1%, and the yield is 89.6%.
Embodiment 2
[0017] Using the same experimental device and the same raw materials as in Example 1, add 500g of pyridine and 340g of water, feed 540g of chlorine gas, and the reaction temperature is 160-180°C. Coke appears on the inner wall of the reactor, and dark red liquid flows out from the outlet of the reactor. Even visible white crystals, obtained 534.6g 2-chloropyridine after 120 minutes of reaction, 198.0g 2.6-dichloropyridine containing a small amount of impurities, the total conversion rate of the reaction was 96.8%, and the yield of 2-chloropyridine was 74.7%. Sex is 77.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com