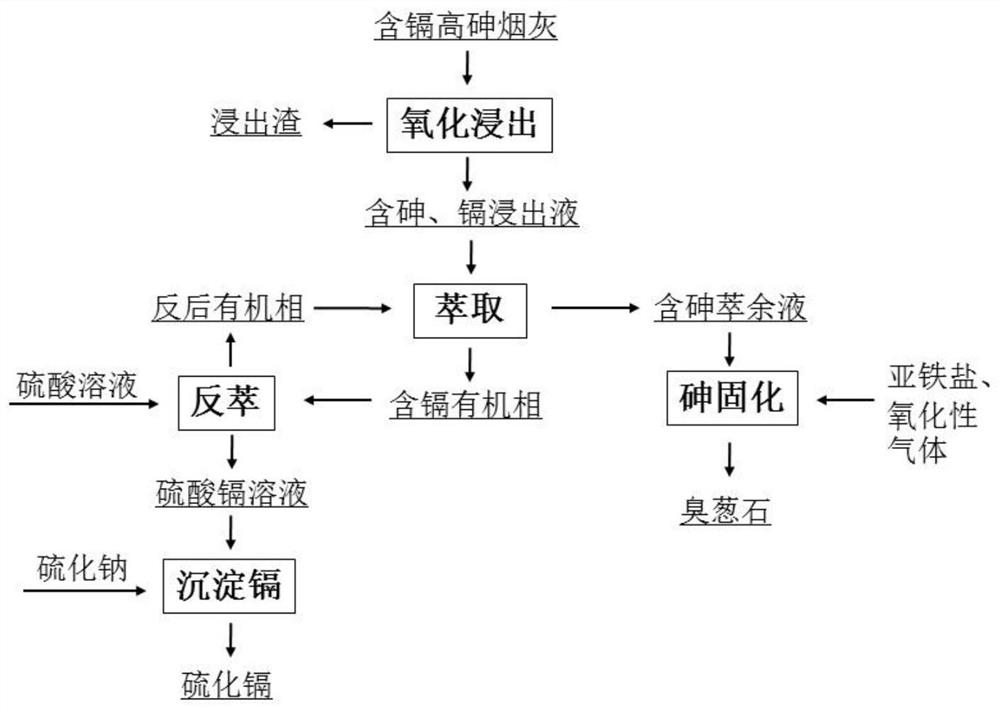
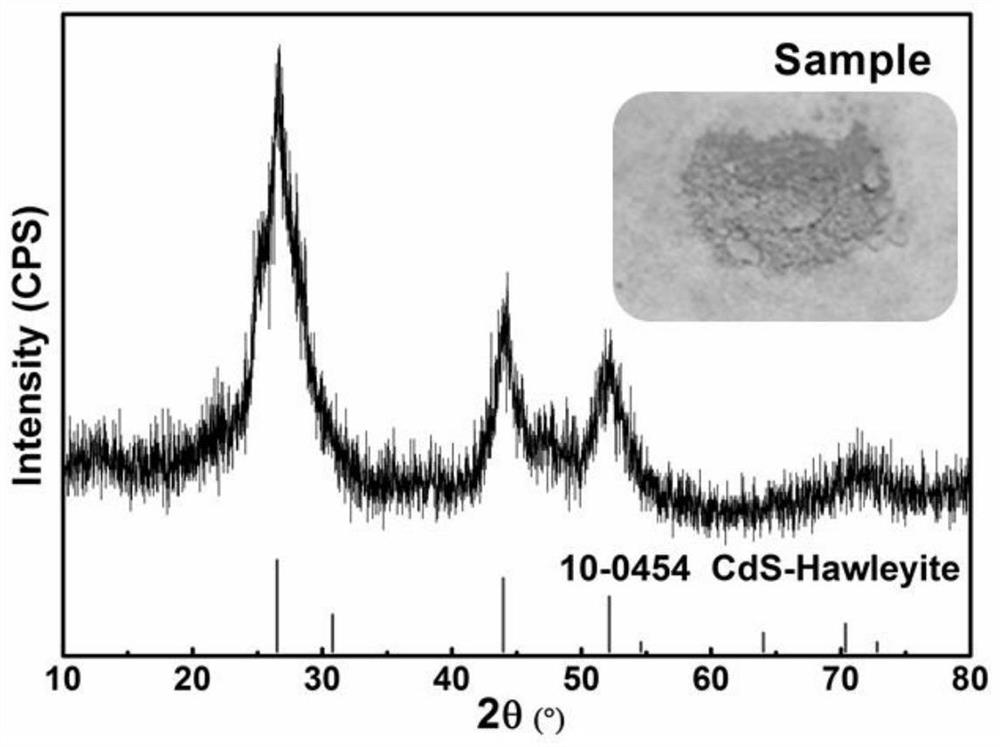
A method for recovering cadmium from cadmium-containing high arsenic soot
A technology of soot and arsenic elements, applied in chemical instruments and methods, improvement of process efficiency, iron compounds, etc., to achieve the effect of avoiding arsine gas and efficient recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take 60ml of 30% hydrogen peroxide (H 2 O 2 The molar concentration is 9.8mol / L) and 340ml of water is added to form a leaching solution, 80 g of the above-mentioned raw materials are added, and the leaching solution containing cadmium and arsenic and the leaching slag containing valuable metals are obtained by stirring for 4h and filtering, and the obtained leaching slag is sent to recycle lead, wherein cadmium The leaching rate of arsenic was 88.2%, and the leaching rate of arsenic was 97.3%. After adjusting the pH of the leaching solution containing cadmium and arsenic elements to 2.7 with sodium hydroxide solution, use P204 (P204: sec-octanol: sulfonated kerosene = 6: 1: 13, water phase: oil phase = 1: 1) to carry out Four-stage countercurrent extraction of cadmium, pH was adjusted to 2.7 with sodium hydroxide solution after each stage of extraction, and then the next stage of extraction was carried out. The extraction time was 15 minutes, and the extraction rate o...
Embodiment 2
[0037] Take 72ml of 30% hydrogen peroxide (H 2 O 2 The molar concentration is 9.8mol / L) and 408ml of water is added to form a leaching solution, 80 g of the above-mentioned raw materials are added, and the leaching solution containing cadmium and arsenic elements and the leaching residue containing valuable metals are obtained by stirring for 4h and filtering, and the gained leaching residue is sent to reclaim lead, wherein cadmium and arsenic are filtered. The leaching rate of arsenic was 90.9%, and the leaching rate of arsenic was 94.8%. After adjusting the pH of the leaching solution containing cadmium and arsenic elements to 2.7 with sodium hydroxide solution, use P204 (P204: sec-octanol: sulfonated kerosene = 15: 1: 34, water phase: oil phase = 1: 1) to carry out Four-stage countercurrent extraction of cadmium, pH was adjusted to 2.7 with sodium hydroxide solution after each stage of extraction, and then the next stage of extraction was carried out. The back-extraction tim...
Embodiment 3
[0039] Take 80ml of 30% hydrogen peroxide (H 2 O 2 The molar concentration is 9.8mol / L) and 320ml of water is added to form a leaching solution, 80 g of the above-mentioned raw materials are added, and the leaching solution containing cadmium and arsenic and the leaching slag containing valuable metals are obtained by stirring and filtering for 3h. The leaching rate of arsenic was 86.2%, and the leaching rate of arsenic was 99.2%. After adjusting the pH of the leaching solution containing cadmium and arsenic elements to 2.7 with sodium hydroxide solution, use P204 (P204: sec-octanol: sulfonated kerosene = 6: 1: 13, water phase: oil phase = 1: 1) to carry out Four-stage countercurrent extraction of cadmium, pH was adjusted to 2.7 with sodium hydroxide solution after each stage of extraction, and then the next stage of extraction was carried out. The extraction time was 15 minutes, and the extraction rate of cadmium was 98.8%. The obtained cadmium-containing organic phase was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com