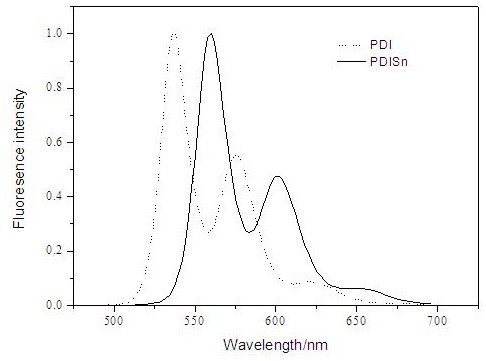
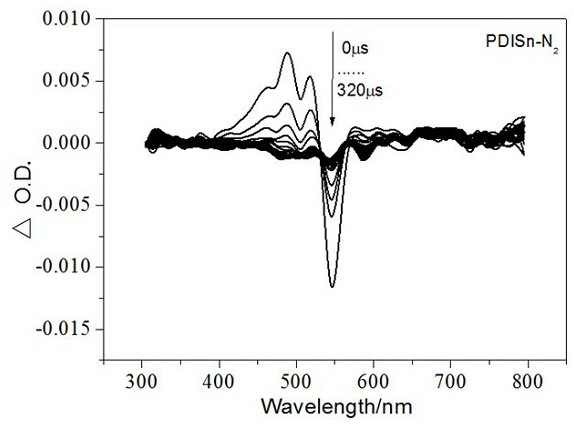
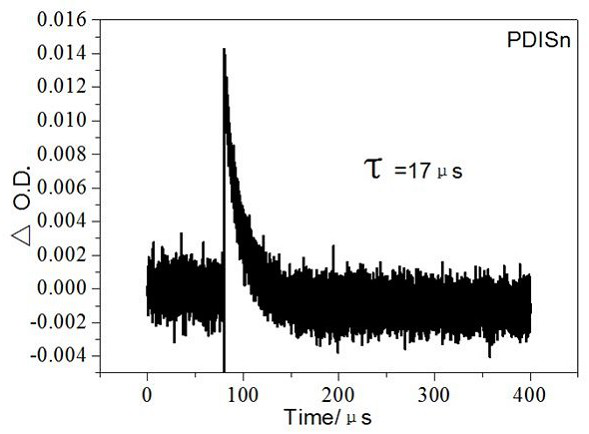
A class of metal tin cyclized perylene imide derivatives and its preparation method and application
A technology of perylene imide and metal tin is applied in the field of organic semiconductor materials to achieve the effects of simple structure, long triplet lifetime and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Weigh 1g of bromoperyleneimide, 1.9g of hexa-n-butylditin, 15mg of dibenzylideneacetone palladium dichloride, and 20mg of trimethylphenylphosphine in a reaction flask, add 5ml of toluene, and Stir for 6 hours. After the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.6 g of product was obtained by column chromatography, with a yield of 48%, HRMS: found 762.2470.
Embodiment 2
[0026]
[0027] Weigh 1g of bromoperyleneimide, 3.36g of hexa-n-butylditin, 12mg of dibenzylideneacetone palladium dichloride, and 16mg of trimethylphenylphosphine in a reaction flask, add 5ml of toluene, and Stir and reflux for 3 hours. After the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.5 g of the product was separated by column chromatography with a yield of 41%. HRMS (MALDI-TOF): Calculated for C54H70N2O4Sn M-, 930.4358, found 930.4310.
Embodiment 3
[0029]
[0030] Weigh 1g of bromoperyleneimide, 3.92g of hexa-n-butylditin, and 5mg of palladium acetate into a reaction flask, add 5ml of 1,4dioxane, and stir and reflux at 120°C for 3 hours. After the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.48 g of product was obtained by column chromatography with a yield of 40%. HRMS (MALDI-TOF): found 3462.1625.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com