Method for analyzing imidazole residues in recombinant human parathyroid hormone for injection
An analytical method and technology of teriparatide, applied in the field of residue analysis, can solve problems such as imidazole-free residue analysis, and achieve the effects of good verification results, good separation, and control of product quality and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] It should be noted that, in the case of no conflict, the embodiments in the present application and the features in the embodiments can be combined with each other. The present invention will be described in detail below in conjunction with examples.
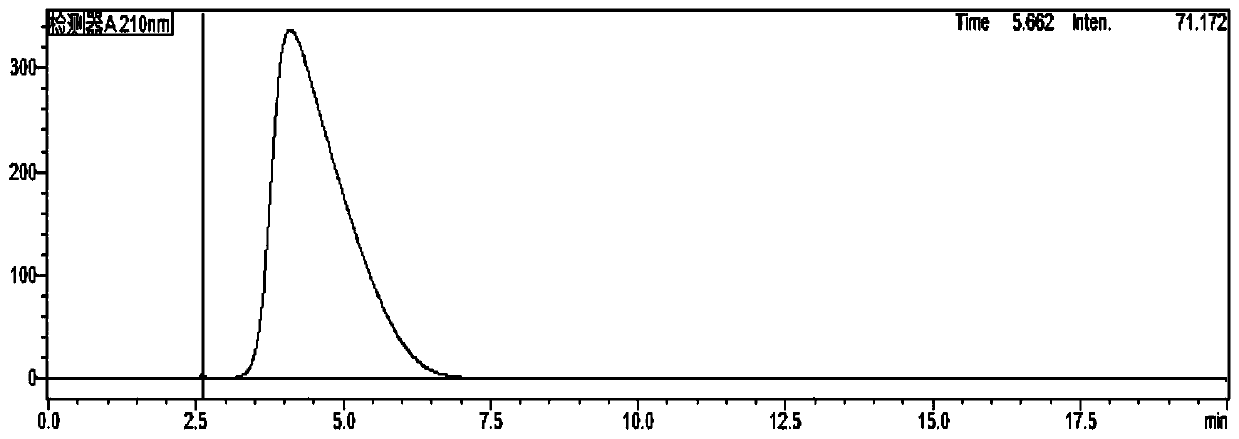
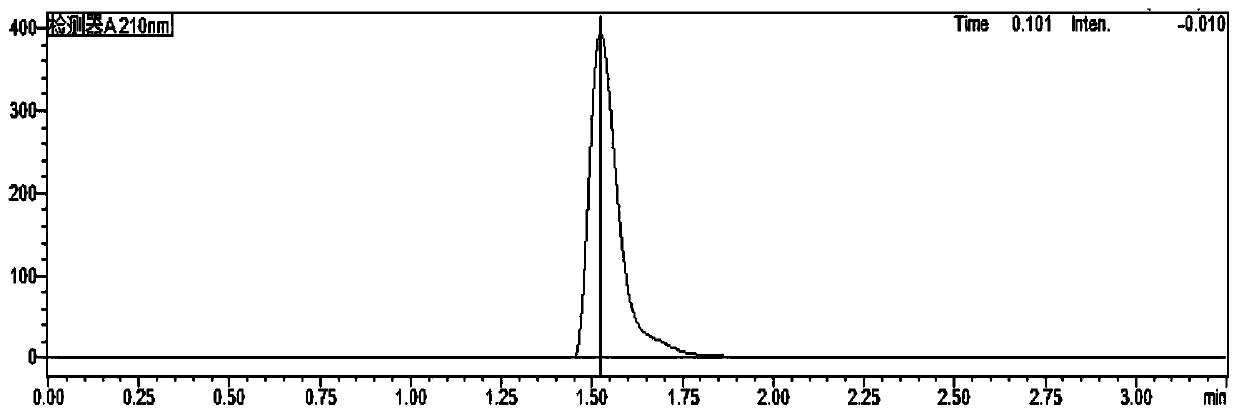
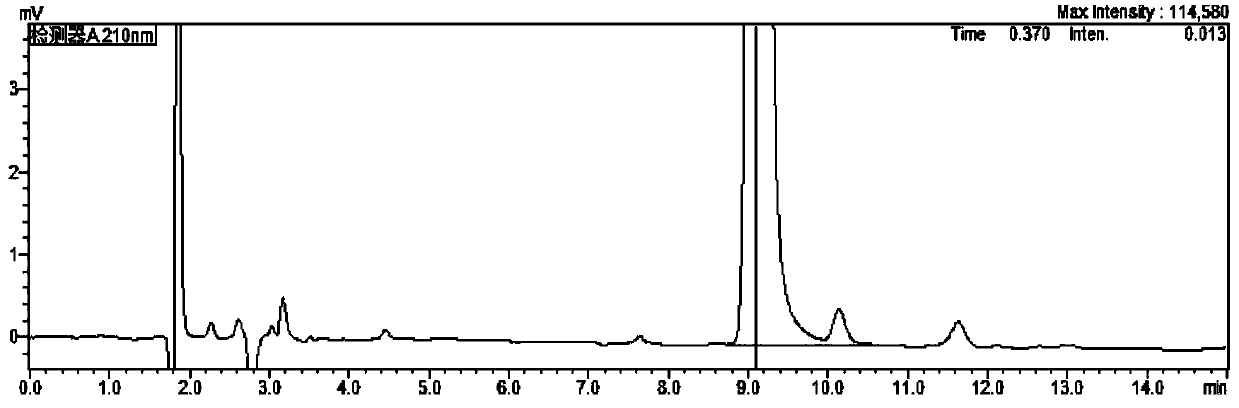
[0034] As mentioned in the background technology, there is no report on the analysis of imidazole residues in biological products such as recombinant human teriparatide for injection in the prior art. In order to make up for this gap, the inventors of the present application used Recombinant human teriparatide for injection was used as the research object, and a detection method for imidazole residues in such biological products was thoroughly studied and developed. details as follows:
[0035] The content of imidazole in the product is low, and imidazole is easily soluble in water, and has a large absorption at the ultraviolet wavelength of about 210nm. The main component of the product (PTH) is a small molecular protei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| injection volume | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com