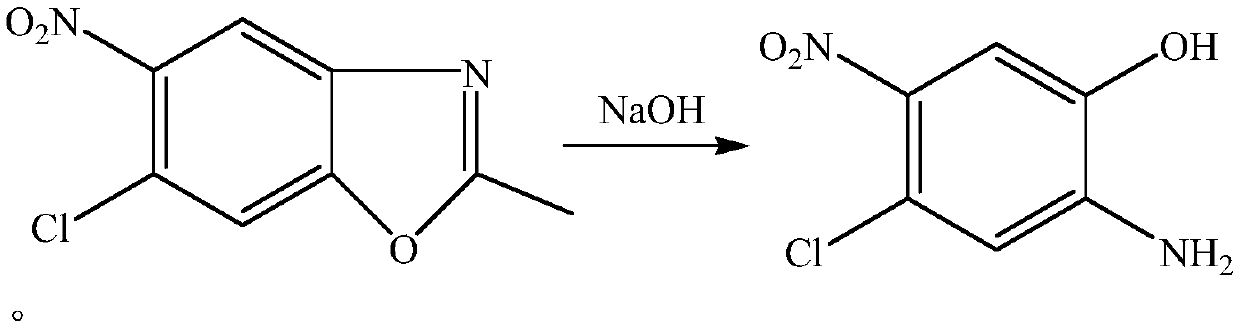
Method for synthesizing 2-amino-4-chloro-5-nitrophenol in micro-channel reactor
A microchannel reactor, nitrophenol technology, applied in chemical instruments and methods, preparation of aminohydroxy compounds, chemical/physical/physical-chemical reactors, etc., can solve production safety and environmental pollution, high cost and low production efficiency and other problems, to achieve the effect of reducing production and by-products, reducing the possibility of safety accidents, and reducing production risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
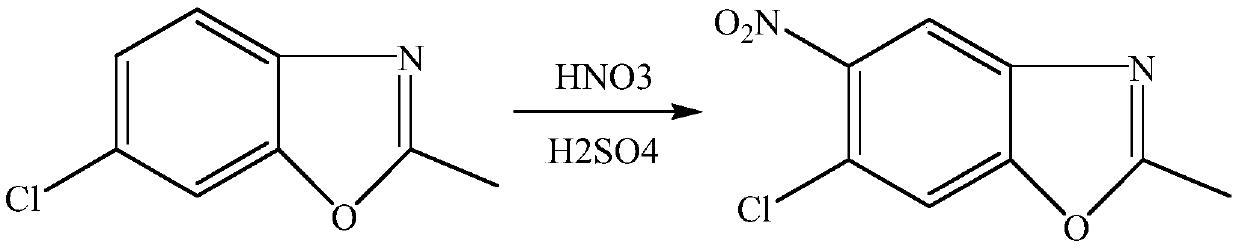
[0018] Example 1-5: Synthesis of 5-chloro-2-methyl-6-nitro-benzoxazole of the present invention: under ice-water bath, 11g of concentrated sulfuric acid and 7g of 65% fume were mixed at a molar ratio of 1.1:1 Slowly mix nitric acid to obtain nitric-sulfur mixed acid, under ice-water bath, dissolve 16.7g (0.1mol) 5-chloro-2-methylbenzoxazole in 98% sulfuric acid at a molar ratio of 1:1, and mix nitric-sulfur mixed acid and the sulfuric acid solution of 5-chloro-2-methylbenzoxazole are pumped into the microchannel reactor respectively, and the flow rate of the sulfuric acid solution of 5-chloro-2-methylbenzoxazole is controlled to be 0.5mL / min in this example, The flow rate of nitric acid mixed acid is 0.3mL / min, the retention time is 10min, and the reaction temperature is 10°C.
[0019] Control the molar ratio of nitric acid to 5-chloro-2-methylbenzoxazole to be 1-1.2:1. After the two liquids are mixed and contacted in the microchannel reactor at 10°C for a moment and react, th...
Embodiment 6-10
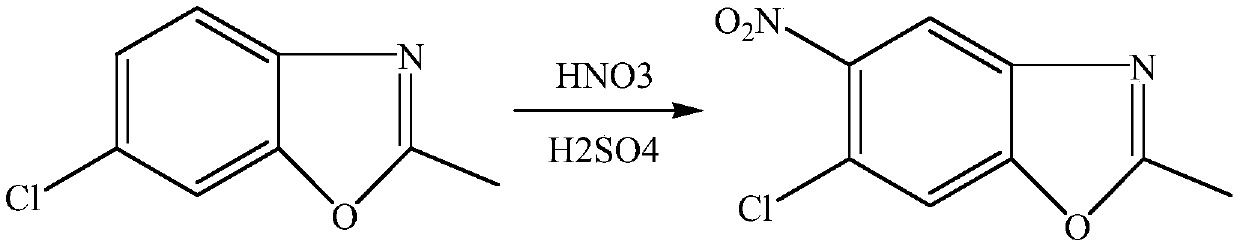
[0023] Embodiment 6-10: The difference between this example and Example 1 is: the synthesis of 5-chloro-2-methyl-6-nitro-benzoxazole in this example: under ice-water bath, the molar ratio is 1 -1.8:1 Slowly mix concentrated sulfuric acid and 65% fuming nitric acid to obtain nitric-sulfur mixed acid, under ice-water bath, mix 16.7g (0.1mol) 5-chloro-2-methylbenzoxan with a molar ratio of 1:1 Azole was dissolved in 98% sulfuric acid, and the sulfuric acid solutions of nitric acid and 5-chloro-2-methylbenzoxazole were pumped into the microchannel reactor respectively. In this case, 5-chloro-2-methylbenzoxazole was controlled. The flow rate of the sulfuric acid solution of oxazole was 0.4 L / min, the flow rate of nitric acid mixed acid was 0.5 mL / min, the retention time was 8 min, and the reaction temperature was 10°C.
[0024] Control the molar ratio of nitric acid to 5-chloro-2-methylbenzoxazole to be 1:1. After the two liquids are mixed and contacted instantly in the microchanne...
Embodiment 1
[0027] Embodiment 11-15: The difference between this example and Example 1 is: the synthesis of 5-chloro-2-methyl-6-nitro-benzoxazole in this example: under ice-water bath, the molar ratio is 1.2 :1 24g vitriol oil and 14g 65% fuming nitric acid are slowly mixed to obtain nitric acid mixed acid, under ice-water bath, 33.4g (0.2mol) 5-chloro-2-methylbenzene will be mixed in molar ratio 1:1-1.5 oxazole is dissolved in 98% sulfuric acid, and the sulfuric acid solution of nitric acid and 5-chloro-2-methylbenzoxazole is pumped into the microchannel reactor respectively. In this example, 5-chloro-2-methyl The flow rate of the sulfuric acid solution of benzoxazole is 0.5mL / min, the flow rate of nitric acid mixed acid is 0.4mL / min, the retention time is 5min, and the reaction temperature is 5°C.
[0028] Control the molar ratio of nitric acid to 5-chloro-2-methylbenzoxazole to be 1:1. After the two liquids are mixed and contacted in a microchannel reactor at 5°C for a moment and react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com