Quinoline chenodeoxycholic acid sensor and its synthesis method and application
A technology of chenodeoxycholic acid and deoxycholic acid, which is applied in the field of quinoline chenodeoxycholic acid sensor and its synthesis, can solve problems such as interference, poor selectivity, and slow response speed, and achieve poor selectivity, easy Operates and detects effects with little interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
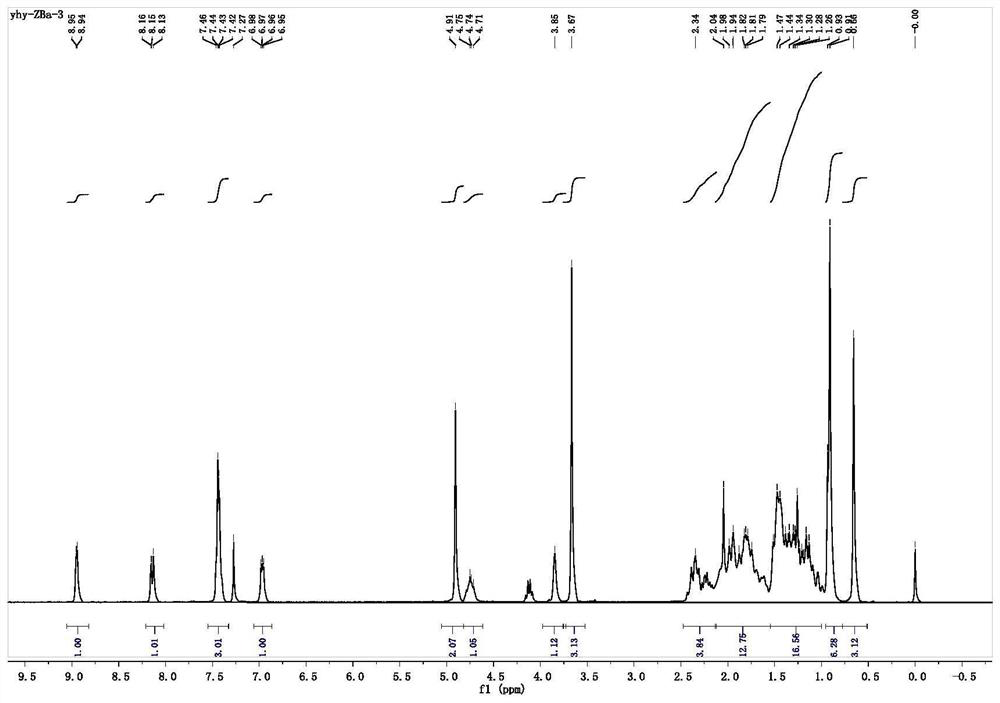
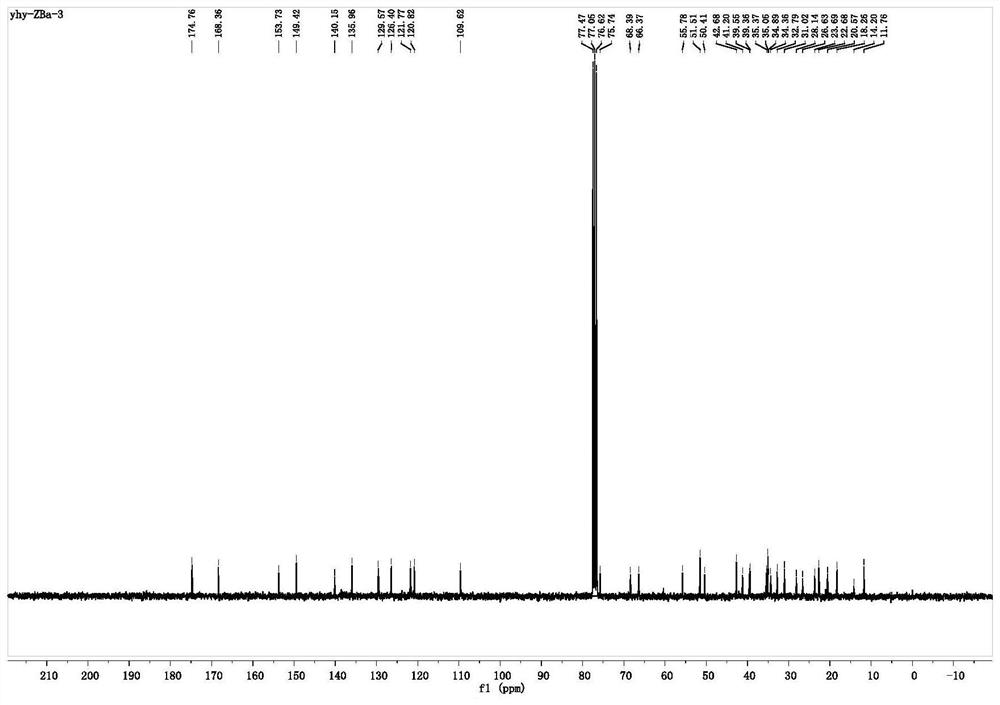
[0038] Synthesis of Compound 1 (CDCA-1): In a 125mL pear-shaped bottle, chenodeoxycholic acid (CDCA) (2.00g, 5.09mmol) was dissolved in 30.00mL of anhydrous methanol, and 1.50mL of concentrated hydrochloric acid was added in The reaction was stirred at room temperature for 6h. The solvent was distilled off under reduced pressure, and the obtained oil was dissolved in 300 mL of dichloromethane, washed with saturated sodium bicarbonate solution, 100 mL was added each time, washed twice, dried with anhydrous magnesium sulfate, and separated by column chromatography (washing Remove agent V 石油醚 :V 乙酸乙酯 =2:1), 1.30 g of compound 1 (CDCA-1) was obtained as a white powder, with a yield of 63%.
[0039] Synthesis of Compound 2 (CDCA-2): In a 125 mL pear-shaped flask, 1.30 g (3.01 mmol) of Compound 1 (CDCA-1) was dissolved in 30 mL of anhydrous CH 2 Cl 2, then add 1.68g (12.03mmol) anhydrous K 2 CO 3 , in an ice bath, 1.05 mL (12.03 mmol) of bromoacetyl bromide in anhydrous CH 2 ...
Embodiment 2
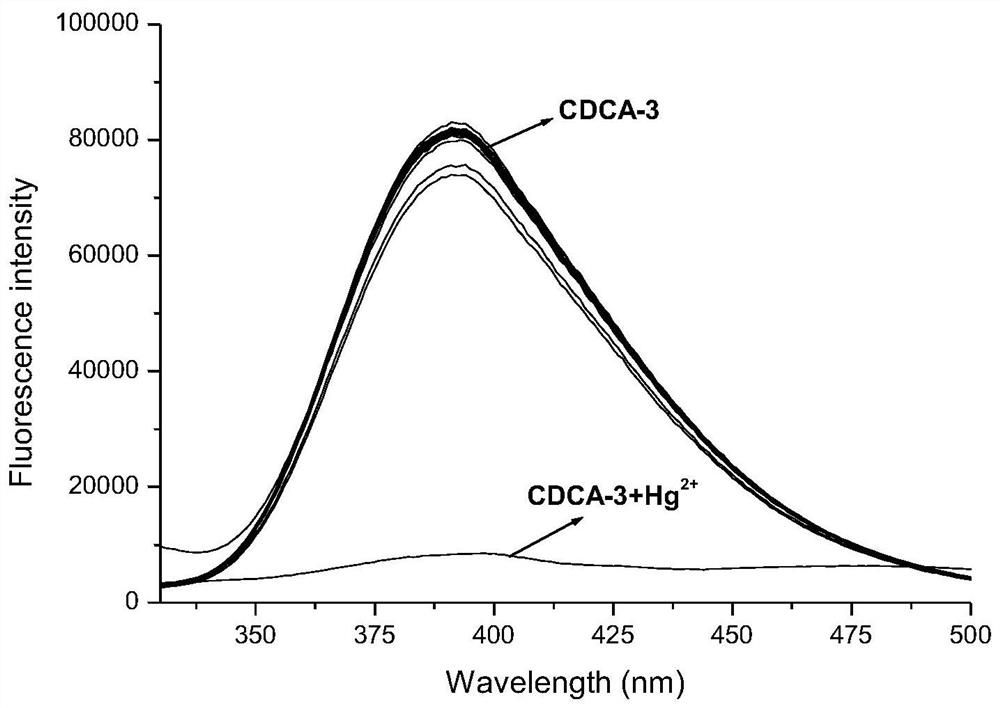
[0044] The quinoline chenodeoxycholic acid sensor (CDCA-3) that embodiment 1 obtains is to Hg 2+ selective test
[0045] A certain amount of quinolinated chenodeoxycholic acid sensor (CDCA-3) was dissolved in acetonitrile-water solution (V 乙腈 :V 水 = 4: 1), 1×10 -5 mol / L stock solution of compound CDCA-3. Take 20 4mL CDCA-3 stock solution, add 20uL successively, the concentration is 2.0×10 -3 mol / L Cr(NO 3 ) 3 .9H 2 O, Ni(NO 3 ) 2 .6H 2 O, Fe(NO 3 ) 3 .9H 2 O, Cd(NO 3 ) 2 .4H 2 O, Sr(NO 3 ) 3 , Pb(NO 3 ) 2 、KNO 3 、LiNO 3 , AgNO 3 , Co(NO 3 ) 2 .6H 2 O, Al(NO 3 ) 3 .9H 2 O, Hg(NO 3 ) 2 .1 / 2H 2 O, ZnSO 4 .7H 2 O, FeSO 4 .7H 2 O, MgSO 4 , CuCl 2 .2H 2 O, MnCl 2 .4H 2 O, CaCl 2 , NaCl, BaCl 2 .2H 2 O solution, the final concentration of metal ions is 1×10 -5 mol / L. The blank is to add 20uL deionized water.
[0046] After shaking well, measure the change of fluorescence spectrum before and after adding metal ions. Test conditions: excit...
Embodiment 3
[0048] The quinolinated chenodeoxycholic acid sensor (CDCA-3) that embodiment 1 obtains is to different concentration Hg 2+ fluorescence detection test
[0049] Add 0.02mL of different concentrations of Hg 2+ solution, mixed evenly, respectively to obtain Hg 2+ The final concentration of ions is solution a of 0mol / L, that is, there is no Hg in solution a 2+ ion, 1×10 - 6 m6ol / L solution b﹑1.5×10 -6 mol / L solution c﹑2×10 -6 mol / L solution d﹑3×10 -6 mol / L solution e﹑4×10 -6 mol / L solution f﹑5×10 -6 mol / L solution g﹑6×10 -6 mol / L solution h﹑7×10 -6 mol / L solution i﹑8×10 -6 mol / L solution j﹑9×10 -6 mol / L solution k﹑1×10 -5 mol / L solution l﹑1.25×10 -5 mol / L solution m﹑1.75×10 -5 mol / L solution n﹑2×10 -5 mol / L solution o﹑1×10 -5 mol / L solution p, for later use. The changes in the fluorescence spectra of the above solutions were tested. Test conditions: excitation wavelength is 295nm, slit: EX is 5nm, EM is 5nm. Test results such as Figure 5 as shown, Figure 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com