Vokapalane-type diterpene derivative, preparation method and application thereof
A technology of vocapane-type and derivatives, which is applied in the field of vocapane-type diterpene derivatives and their preparation, and can solve the problems of no maintenance effect, side effects, and slow onset of immunosuppressants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 20.0kg of hematoxylinum seeds, peeled to obtain 9.0kg of seed kernels, extracted with 75% ethanol for 3 times, the ratio of material to liquid is 1:8, each time 2h, the solvent is recovered under reduced pressure, the extract is dispersed with distilled water, followed by It was extracted three times with equal volumes of chloroform, ethyl acetate and n-butanol to obtain 214.0 g of chloroform layer extract.
[0055] The obtained chloroform layer extract 214.0g was separated by silica gel open column chromatography, and the mobile phase was eluted with a gradient of dichloromethane: methanol (100:0-1:1), and the obtained fractions were analyzed by silica gel thin layer chromatography, and the same was combined. After fractionation, 10 fractions of eluate were obtained.
[0056] Fraction 18.8 g of dichloromethane:methanol=100:2 was separated. Using silica gel column chromatography, eluting with a gradient of petroleum ether:ethyl acetate, at 15:1, the yellow solid was se...
Embodiment 2
[0058] Screening of in vitro anti-inflammatory activities of diterpene derivatives of vokaparaane
[0059] (1) Cell culture
[0060] RAW264.7 cells in DMEM, 5% FBS, 37 °C, 5% CO 2 cultured under conditions.
[0061] (2) Activity test
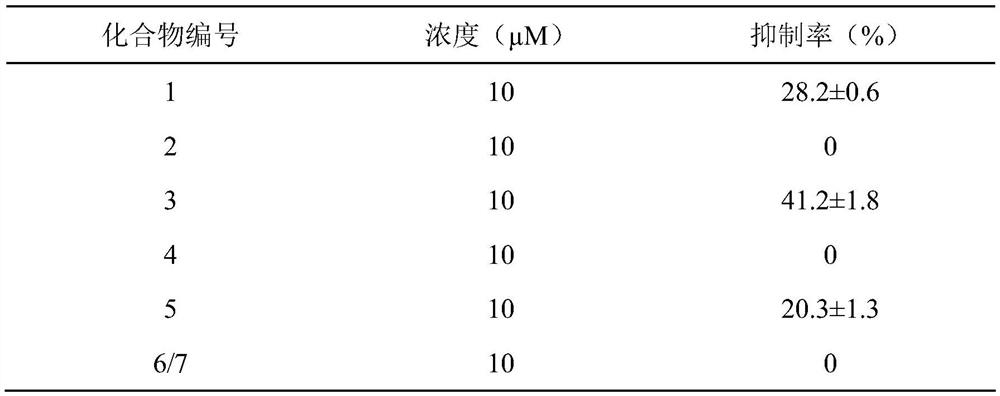
[0062] To test the in vitro anti-inflammatory activity of the test drugs, the compounds at a final concentration of 10 μM were incubated with LPS-stimulated RAW264.7 cells for 6 h, and then the inflammatory cytokines IL-1β, IL-6 and TNF-α were detected by q-PCR. alpha mRNA levels.
[0063] The experimental results are shown in Table 1-3.
[0064] Table 1 The effect of vokapalane-type diterpene derivatives on LPS-induced expression of IL-1β mRNA in RAW264.7 cells
[0065]
[0066] Table 2 The effect of vokapalane-type diterpene derivatives on LPS-induced expression of pro-inflammatory factor IL-6 mRNA in RAW264.7 cells
[0067]
[0068] Table 3 The effect of vokapalane-type diterpene derivatives on LPS-induced TNF-α mRNA expression in...
Embodiment 3
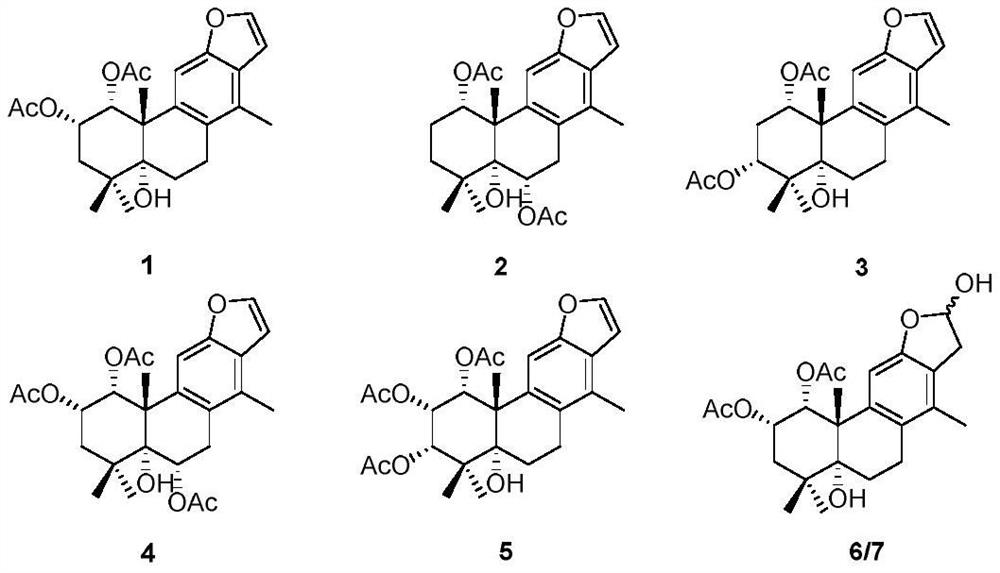
[0072] The effect of compound 3 on dextra sulfate sodium (DSS)-induced ulcerative colitis in mice was tested.
[0073] (1) Model establishment
[0074] First, 6-8 weeks old C57BL / 6 mice (20±2g) were randomly divided into 5 groups, 5 mice in each group, namely: Normal group, DSS group, compound 3 group (10mg / kg) and dexamethasone group (Dex) (1 mg / kg). DSS with a concentration of 3% was prepared with distilled water, and the model group and each administration group drank freely for 7 days. Then, the model group and each administration group were changed to distilled water to drink freely for 3 days, and the Normal group was given distilled water to drink freely for 10 days. Mice were sacrificed every day. From the first day of modeling to the tenth day of sacrifice, compound 3 solution (compound 3 was first dissolved in DMSO and then suspended in PBS, wherein DMSO:PBS=1:100) was administered by intraperitoneal injection. The DSS group was intraperitoneally injected with DMS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com