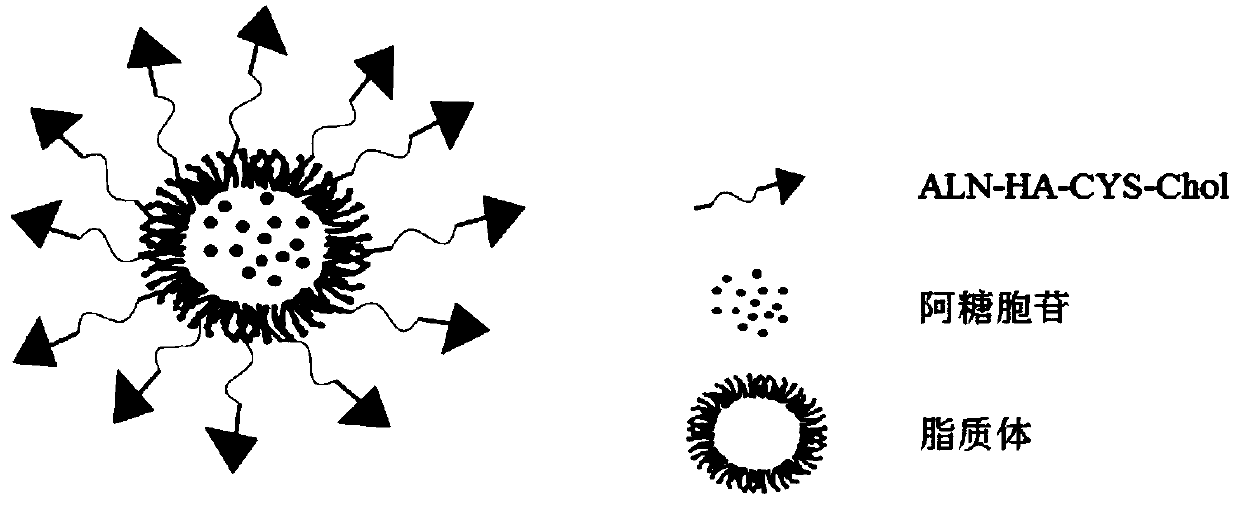
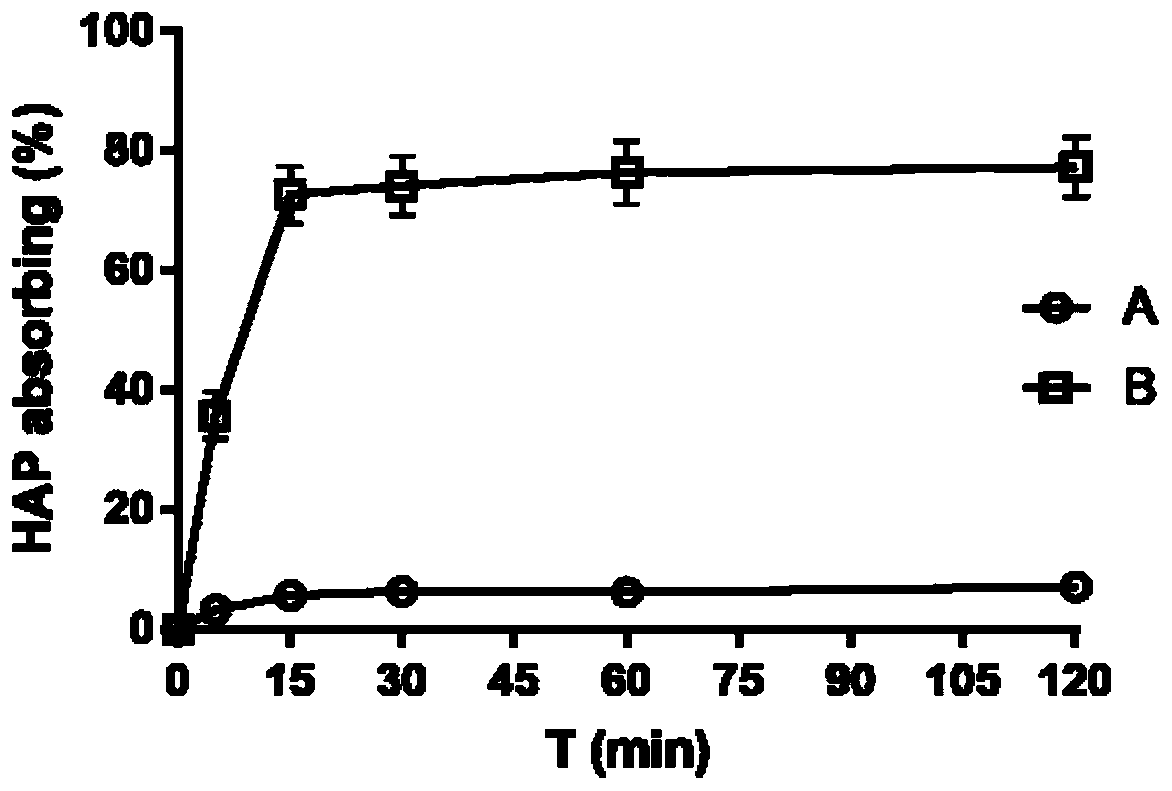
Glutathione sensitive bone targeted liposome and preparation method thereof
A technology of glutathione and liposomes, which is applied in the direction of liposome delivery, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of short half-life, low blood flow, and reduced drug treatment. Efficiency and other issues to achieve the effect of reducing toxic side effects and increasing long circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh 160 mg of soybean lecithin, 16 mg of cholesterol, and 5 mg of PEG into a 100 mL eggplant-shaped bottle, add 20 mL of dichloromethane to dissolve, spin dry at 48 °C and 50 r / min, and then add 10 mL of water The membrane was washed, the probe was sonicated for 30 min to obtain blank liposomes, and then co-incubated with cytarabine solution (40 mg / mL, 10 mL) and dialyzed to obtain cytarabine liposomes. Take 1mL cytarabine liposomes in a vial, and then prepare 2 mg / mL bone-targeting material ALN-HA-CYS-Chol, heat and co-incubate in a vial, and the incubation temperature should be higher than that of the liposome phase. Change the temperature, and after it cools down, pass through 0.45 μm and 0.22 μm filters in turn to obtain bone-targeted liposomes.
Embodiment 2
[0043] Weigh 160 mg of soybean lecithin, 16 mg of cholesterol, 5 mg of PEG, and 20 mg of bone-targeted substance in an eggplant-shaped bottle, add 20 mL of dichloromethane to dissolve, spin dry at 48 °C at 50 r / min, and then Add 10 mL of water to wash the membrane, sonicate the probe for 30 min to obtain blank bone-targeted liposomes, then co-incubate with cytarabine solution (40 mg / mL, 10 mL), and finally dialyze to remove free arabinose Cytidine yields bone-targeted cytarabine liposomes.
Embodiment 3
[0045] Weigh 160 mg of soybean lecithin, 16 mg of cholesterol, 5 mg of PEG, and dissolve 20 mg of bone-targeting substance in tetrahydrofuran, then slowly drop it into 10 mL of PBS and keep stirring, after the tetrahydrofuran volatilizes, you can get Bone-targeted blank liposomes, and finally co-incubated cytarabine aqueous solution (40 mg / mL, 10 mL) with blank liposomes and dialyzed to obtain cytarabine bone-targeted liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com