Combination cancer therapy
A cancer and composition technology, applied in the field of conjugates of cytarabine and aspartic acid, can solve the problem of high toxicity of cytarabine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
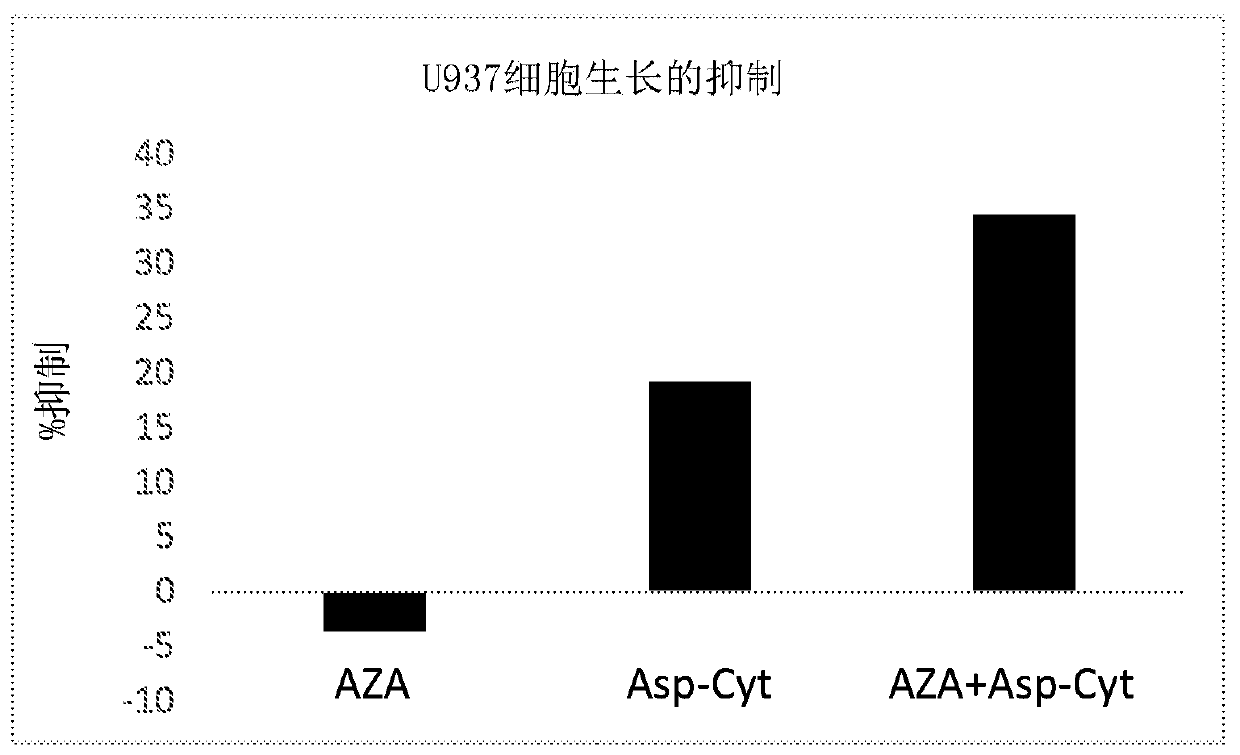
[0413] Effects of Asp-cytarabine / BST-236 and azacitidine (Vidaza) on the proliferation and survival of U937 cells
[0414] U937 human blood cancer cells were cultured in RPMI supplemented with 10% FCS. cells at 1x10 5 Cells / well were seeded in a 96-well plate in a total volume of 250 μl. Azacitidine (AZA) was added to the cell culture at 5 different concentrations: 0, 100, 250, 1000, 5000 nM. Asp-cytarabine, hereafter also referred to as BST-236, was added to the cultures at a concentration of 250 nM. All groups were analyzed in triplicate. at 37°C with 5% CO 2 After 72 hours of incubation at 0°C, cells were harvested, stained with propidium iodide (PI), and immediately read by FACS. The number and percentage of live (PI-negative) cells and the number and percentage of dead (PI-positive) cells in the cultures were determined by FACScalibur using CellQuest software. Calculate percent inhibition.
[0415] Table 2. Percent growth inhibition of U937 cells treated with Asp-c...
example 2
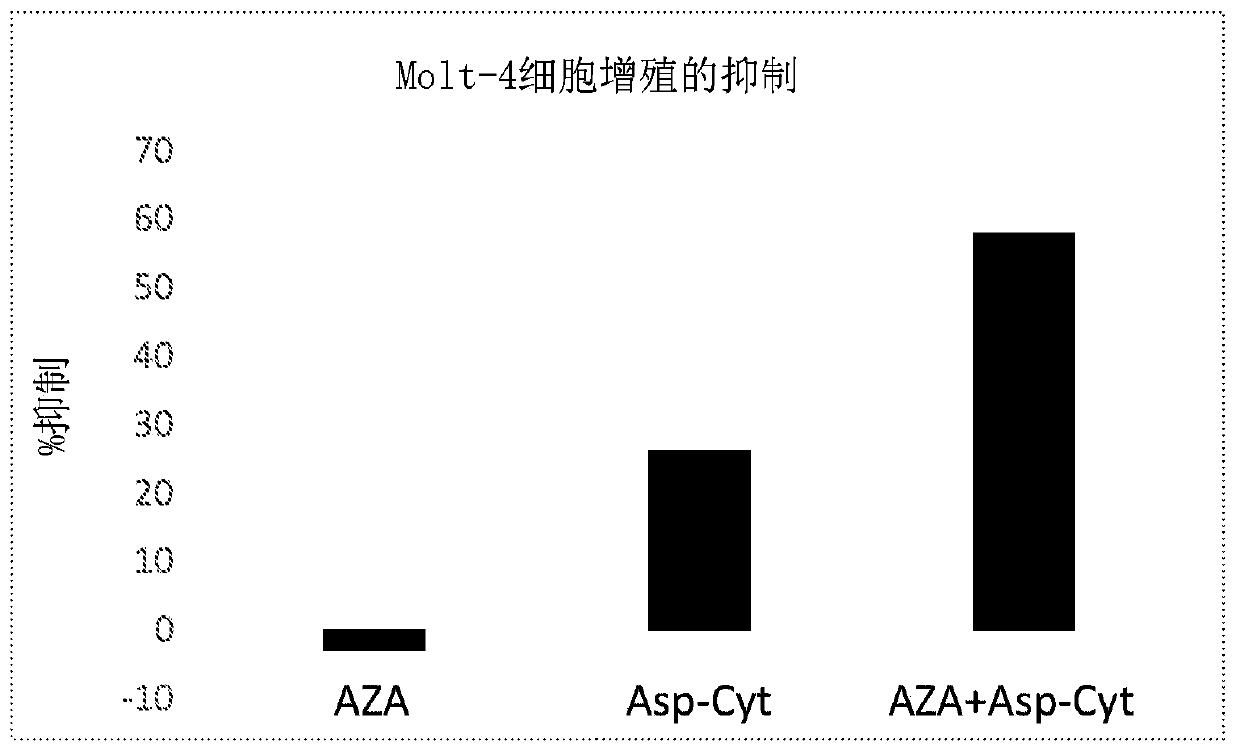
[0422] Effects of Asp-cytarabine and azacitidine on the proliferation of Molt-4 cells
[0423] The Molt-4 human leukemia cell line was obtained from ATCC. Cells were grown in RPMI medium containing 10% FBS and 1% glutamine. Cells were seeded in 96-well plates at 50,000 cells / ml, 0.2ml / well. Test substances were diluted in PBS and added at final concentrations of 0.1 nM to 10 μM in a volume of 20 μl. The study was performed in triplicate. PBS was used as a control. Incubate the plate at 37 °C with 5% CO 2 Incubate for 72 hours. At the end of the treatment period, an MTT assay using the MTT reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was performed. MTT was added to each well at a concentration of 5 mg / ml in a volume of 0.02 ml. Plates were incubated at 37°C for 3 hours. The plate was centrifuged at 3500 rpm for 5 minutes and the supernatant was aspirated. The pellets containing MTT crystals were each dissolved in 0.2 ml DMSO. Absorbance was m...
example 3
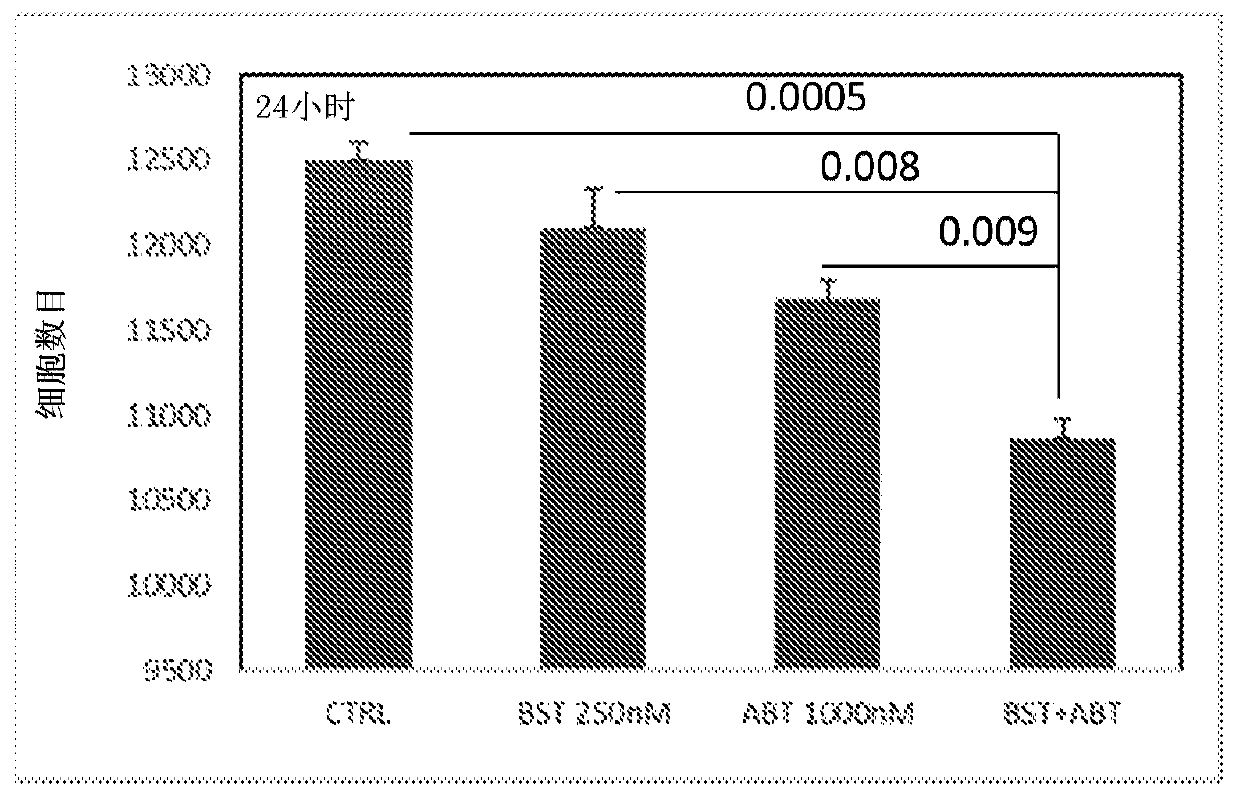
[0432] Effects of Asp-cytarabine and ABT-199 (venetoclax) on the proliferation and survival of U937 cells
[0433] U937 cells were cultured in RPMI supplemented with 10% FCS, and 1x10 5 Cells / well were seeded in a 96-well plate in a total volume of 250 μl. ABT-199 was added to cell cultures at 3 different concentrations: 0, 250 and 1000 nM. Asp-cytarabine was added to the cultures at a concentration of 250 nM. All groups were analyzed in triplicate. at 37°C with 5% CO 2 After 24 hours of incubation at 0°C, cells were harvested and stained with propidium iodide (PI) and read immediately by FACS. The number and percentage of live (PI-negative) cells and the number and percentage of dead (PI-positive) cells in the cultures were determined by FACScalibur using CellQuest software. Calculate percent inhibition.
[0434] Such as image 3 As shown in , treatment of human hematological cancer cells with the combination of Asp-cytarabine and ABT-199 for 24 hours resulted in a sig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com