Synthesis method of cobalt-molybdenum bimetallic sulfide for supercapacitor electrode material
A technology for supercapacitors and electrode materials, which is applied in the manufacture of hybrid capacitor electrodes and hybrid/electric double-layer capacitors. It can solve unsatisfactory problems and achieve the effects of improving specific capacitance, good electrochemical performance, and high reversibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0038] In the synthetic method of the cobalt-molybdenum bimetallic sulfide that is used for the supercapacitor electrode material of this example, at first, cobalt chloride hexahydrate (CoCl 2 ·6H 2 O) and deionized water are mixed and dissolved in a ratio of 0.002:1 to obtain solution A, and then the morphology regulator ammonium fluoride, sodium tetrathiomolybdate (Na 2 MoS 4 ) and deionized water were mixed and dissolved according to the mass ratio of 0.001:0.002:1 to obtain solution B. Then, mix solution A and solution B in equal volumes and carry out hydrothermal reaction. The reaction temperature is 100°C and the reaction time is 6 hours. Under the conditions of 0.8KPa and 60°C, vacuum drying was carried out for 10 hours to obtain cobalt-molybdenum bimetallic sulfides.
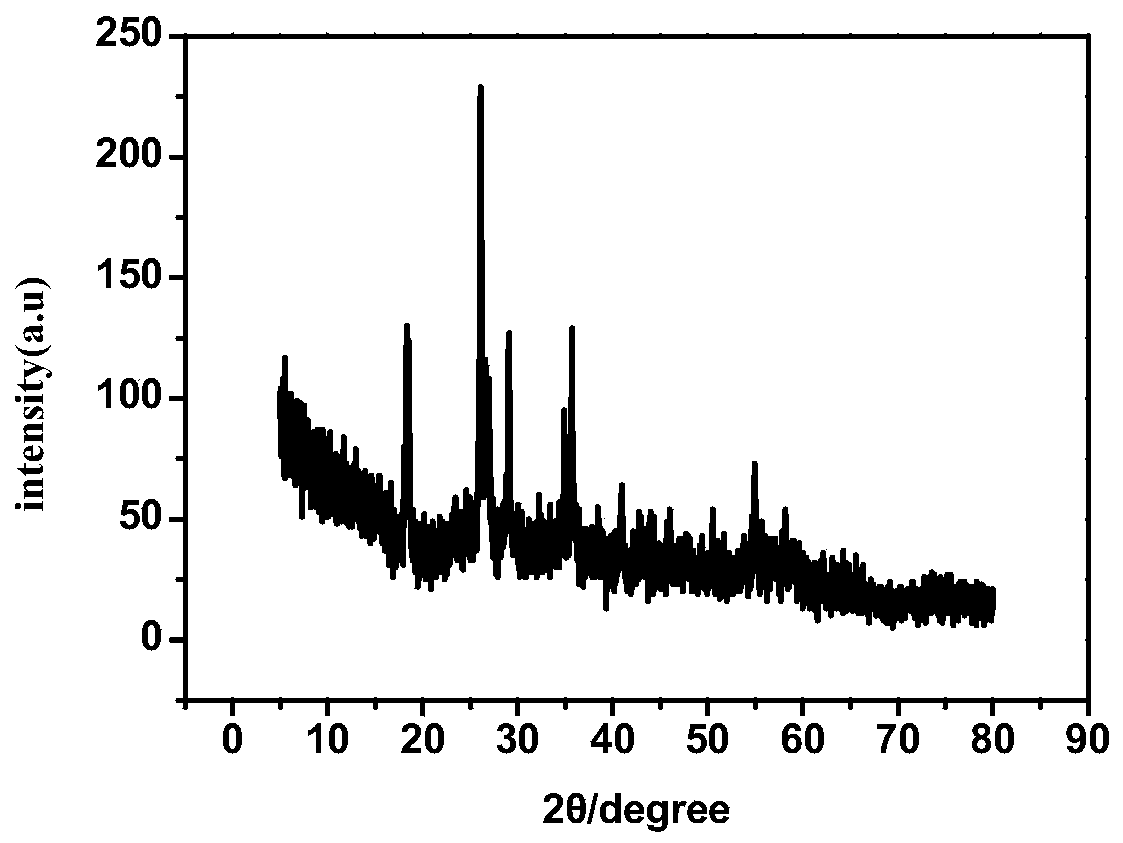
[0039] The cobalt-molybdenum bimetallic sulfide synthesized by this preparation example, after testing its XRD pattern is as follows figure 1Shown in (test conditions are Bruker D8 and BDX3300 X-ray ...
preparation example 2
[0044] In the synthetic method of the cobalt-molybdenum bimetallic sulfide that is used for supercapacitor electrode material of this example, at first, cobalt nitrate (Co(NO 3 ) 2 ) and deionized water are mixed and dissolved at a ratio of 0.003:1 to obtain solution A, and then the morphology regulator sodium lauryl sulfate, potassium tetrathiomolybdate (K 2 MoS 4 ) and deionized water were mixed and dissolved according to the mass ratio of 0.001:0.003:1 to obtain solution B. Then, mix solution A and solution B in equal volumes and carry out hydrothermal reaction. The reaction temperature is 120°C and the reaction time is 8 hours. Under the conditions of 0.8KPa and 60°C, vacuum drying was carried out for 10 hours to obtain cobalt-molybdenum bimetallic sulfides.
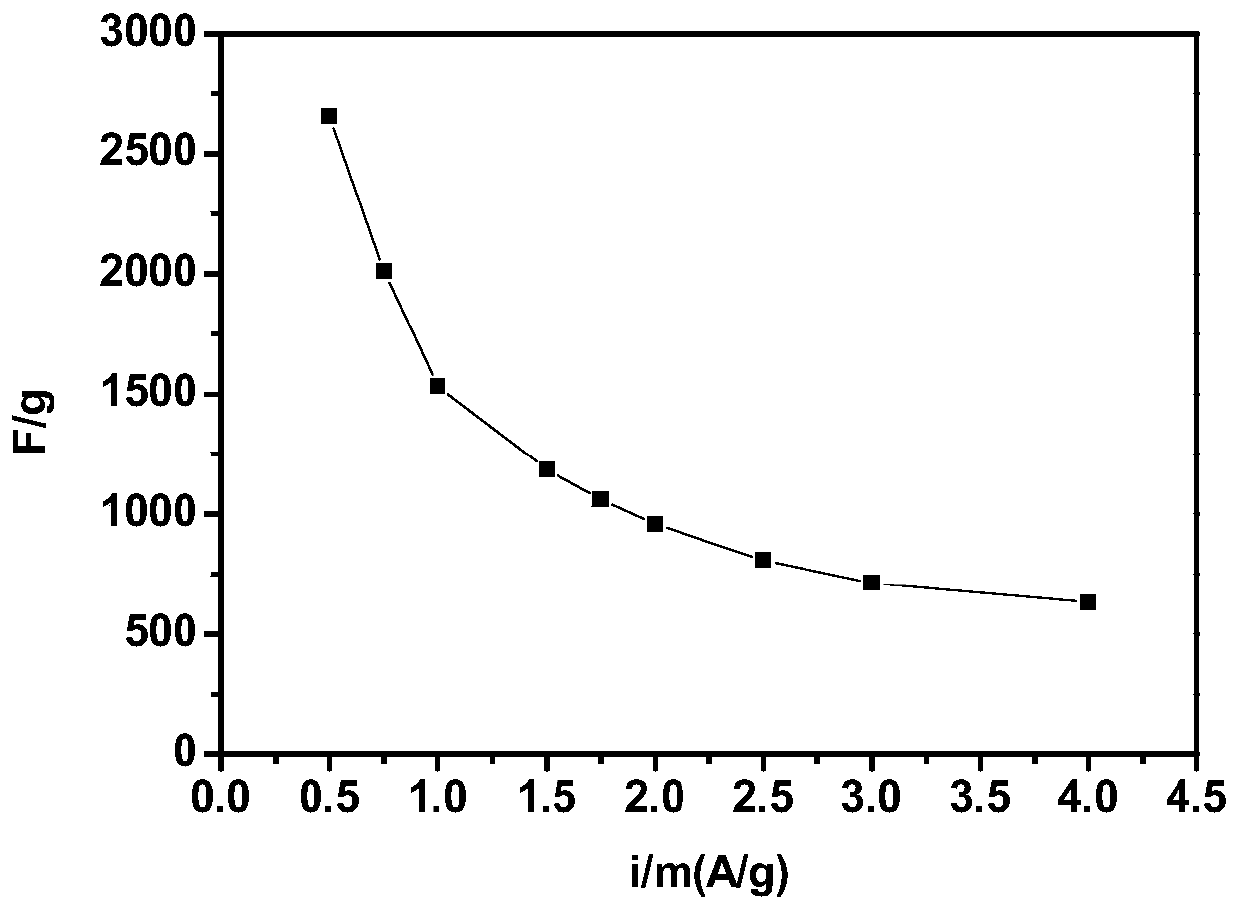
[0045] The electrode material of the cobalt-molybdenum bimetallic sulfide synthesized in this example was subjected to the same electrochemical test as in Example 1. After testing, its specific capacitance value r...
preparation example 3
[0047] In the synthetic method of the cobalt-molybdenum bimetallic sulfide that is used for the supercapacitor electrode material of this example, at first, cobalt bromide (CoBr 2 ) and deionized water are mixed and dissolved at a ratio of 0.003:1 to obtain solution A, and then the morphology regulator hexadecyltrimethylammonium bromide, ammonium tetrathiomolybdate ((NH 4 ) 2 MoS 4 ) and deionized water were mixed and dissolved according to the mass ratio of 0.002:0.004:1 to obtain solution B. Then, mix solution A and solution B in equal volumes and carry out hydrothermal reaction. The reaction temperature is 140°C and the reaction time is 10 hours. Under the conditions of 0.8KPa and 60°C, vacuum drying was carried out for 10 hours to obtain cobalt-molybdenum bimetallic sulfides.
[0048] The same electrochemical test as in Example 1 was performed on the cobalt-molybdenum bimetallic sulfide electrode material synthesized in this example. After testing, its specific capacita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacitance value | aaaaa | aaaaa |
| Specific capacitance value | aaaaa | aaaaa |
| Specific capacitance value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com