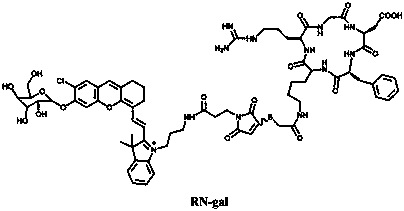
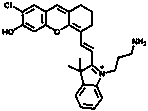
Near-infrared fluorescent probe targeting tumor cells and activated by beta-galactosidase and preparation method thereof
A technology of galactosidase and fluorescent probe, which is applied in the field of targeted small molecule fluorescent probe compounds, can solve the problems of low specificity and achieve the effects of increased fluorescence intensity, wide application range and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for preparing a fluorescent probe compound for β-galactosidase detection, the steps comprising:
[0041] 1) Synthesis of Compound 1:
[0042] Dissolve 2-chloro-1-formyl-3-(hydroxymethylene) compound (1.2 mmol, 1 eq) and indole derivative (3 mmol, 2.5 eq) in 30 mL butanol / benzene (5:2 ) solution, after reflux and stirring, remove the water with a water separator. The reaction solution was further stirred at 80°C for 8 hours. After removing the solvent by evaporation in vacuo, the residue was purified by silica gel column chromatography using CH 2 Cl 2 / CH 3 OH (20:1 to 1:1) was used as the eluent to obtain compound 1 as a green solid in 70% yield.
[0043] 2) Synthesis of Compound 2:
[0044] To the ethanol solution of compound one (0.5 mmol, 1 eq), di-tert-butyl carbonate (1.2 mmol, 2.4 eq) and TEA (1.2 mmol, 2.4 eq) were added, and the mixture was kept stirring at room temperature for 3 hours. The solvent was evaporated under reduced pressure, and the r...
Embodiment 2
[0056] Time-response changes of probe compound RN-gal to β-gal UV absorption and fluorescence emission
[0057] Take the RN-gal synthesized in Example 1 to prepare a 5 μM probe solution, add β-gal (5 U / mL) standard solution, and measure its ultraviolet absorption and fluorescence properties. The absorption peak at 608 nm decreased gradually, and a new absorption peak appeared at 692 nm, accompanied by a red shift of the UV-vis absorption. With 625 nm as the excitation light, the fluorescence emission at 710 nm also increased with time, and reached the maximum fluorescence intensity after 60 minutes. Through fluorescence synchronous scanning, the maximum fluorescence at 710 nm of the probe RN-gal activated by β-gal was about 240 times that before the reaction.
Embodiment 3
[0059] Measurement of the Fluorescence Linear Range of the Probe Compound RN-gal
[0060] Take the fluorescent probe solution (5 μM) in Example 2, add β-gal (0-10 U / mL) respectively, and perform fluorescence detection (λ ex =625 nm), indicating that the probe exhibits a linear relationship in the range of β-gal concentration 0-5 U / mL, and the linear correlation coefficient is R 2 =0.99863.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com