Absorbent for emergency treatment of indoor mercury leakage and preparation method thereof
The technology of an adsorbent and a treatment method is applied in the field of an adsorbent for mercury leakage in an emergency disposal room and its preparation field, which can solve problems such as mercury leakage and achieve the effect of excellent adsorption performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1ZnS / TiO 2 Sorbent preparation
[0043] (1) Zinc nitrate solution is mixed with titanium dioxide, stirred for 3h, wherein, the mass ratio of the dosage of zinc nitrate and titanium dioxide is 1:5;
[0044] (2) Rotate the mixed solution obtained in step (1), dry the solid obtained by rotary evaporation, and then calcinate the solid at 500 ° C to obtain ZnO / TiO 2 Adsorbent;
[0045] (3) Get 250mg of ZnO / TiO prepared in step (2) 2 The adsorbent is placed in a fixed-bed reactor, and hydrogen sulfide is passed through at 300°C for 1 hour (flow rate 500mL / min) to obtain ZnS / TiO 2 Adsorbent, labeled as Adsorbent A.
Embodiment 2
[0057] Example 2 Adsorption of gaseous zero-valent mercury
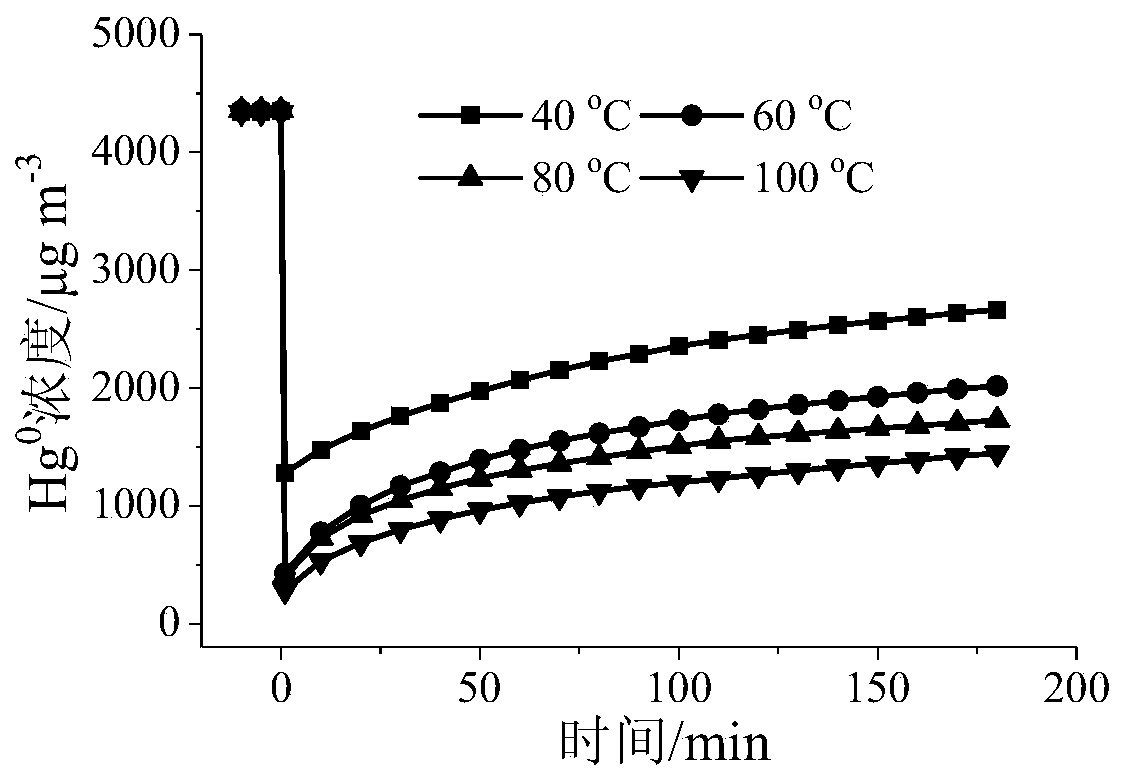
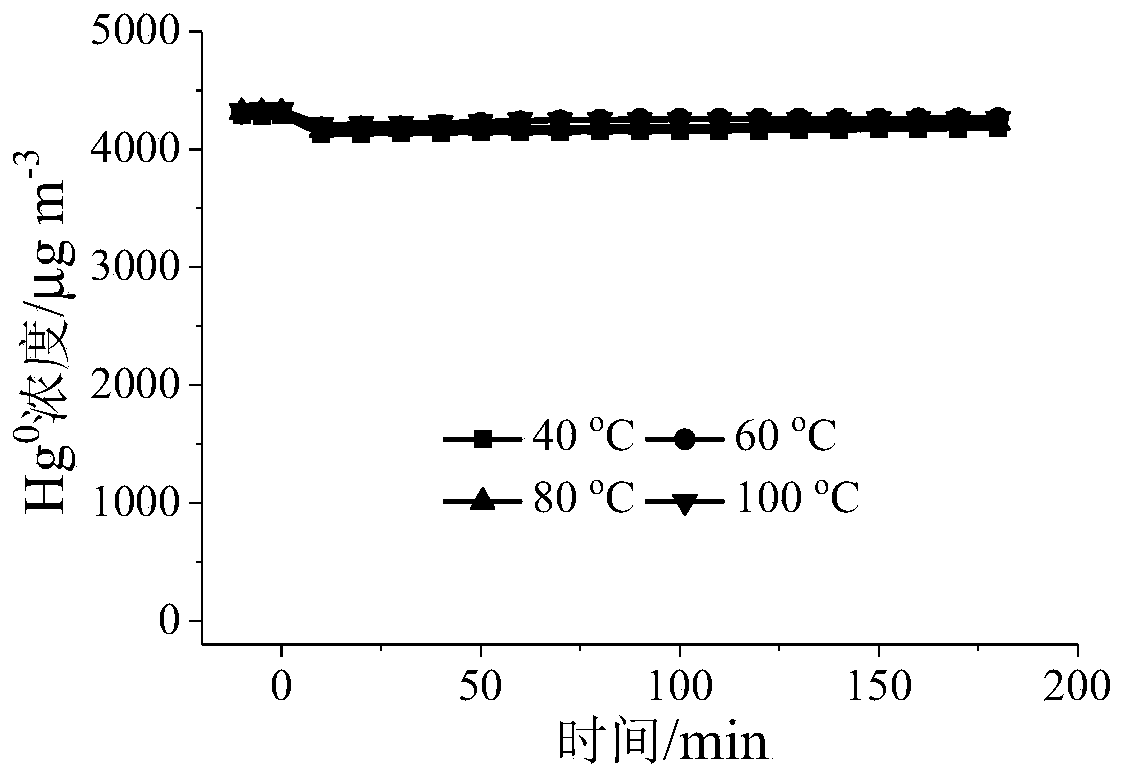
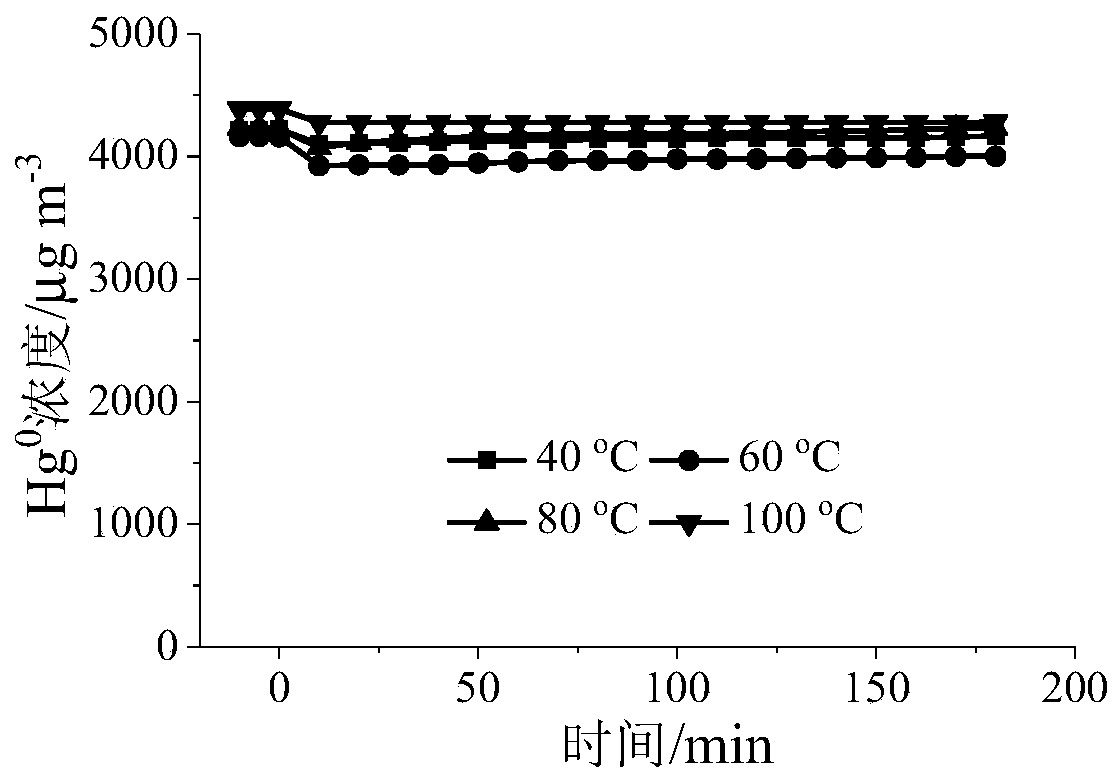
[0058] Grinding and sieving the adsorbents A, B, C, and D prepared in Example 1 and Comparative Examples 1, 2, and 3, and taking 40-60 mesh particles to perform gaseous zero-valent mercury adsorption performance on a fixed-bed reactor Evaluation: The amount of adsorbents A, B, C, and D is 50 mg, and the simulated gas composition is: [Hg 0 ]=4350μgm -3 , nitrogen flow = 300mLmin -1 , airspeed=3.6×10 5 cm 3 g -1 h -1 , reaction temperature = 40 ~ 100 ° C, adsorption time = 3h.
[0059] Determination of reactor outlet Hg 0 concentration, and calculate the Hg 0 The adsorption capacity and penetration rate are shown in Table 1.
[0060] Table 1 Hg of different samples 0 Adsorption amount (μg) and penetration rate
[0061]
[0062] It can be seen from Table 1 that under the same reaction conditions, the adsorption of adsorbent A to Hg 0 The adsorption performance of is significantly better than that of adso...
Embodiment 3
[0064] A certain amount of used adsorbent A was placed on a fixed-bed reactor, and the mercury stability on the surface of adsorbent A was investigated by means of temperature-programmed desorption.
[0065] Adsorbent mass = 50mg; air flow = 700mL min -1 ;50-500°C temperature program (10°C / min), Hg 0 Concentrations were determined using a Lumex R915M mercury analyzer. The mercury temperature-programmed desorption of adsorbent A under air conditions after use is as follows: Figure 5 Shown:
[0066] From Figure 5 It can be seen that a mercury desorption peak appeared under air conditions, indicating that the HgS adsorbed on the surface of adsorbent A could be thermally decomposed. And when the temperature is lower than 100 °C, there is almost no Hg 0 is thermally decomposed, indicating that at room temperature Hg 0 It can be stably adsorbed on the surface of adsorbent A in the form of HgS and will not be released again.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com