A magnetic regenerable adsorbent for adsorbing gaseous zero-valent mercury and its preparation method
An adsorbent and magnetic technology, applied in the field of magnetic renewable adsorbent and preparation, can solve the problems of poor thermal stability and unfavorable recycling of maghemite sulfide, and achieve excellent adsorption performance, good thermal stability and simple separation method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
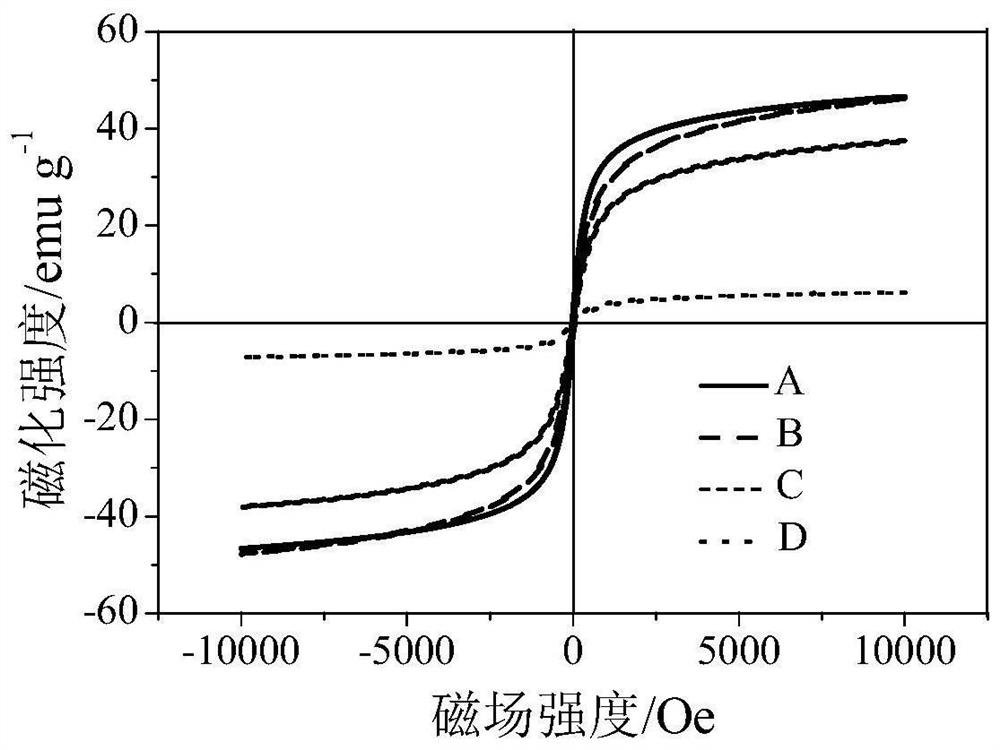
[0040] Example 1MoS x @γ-Fe 2 o 3 Sorbent preparation
[0041] (1) 20g / L phosphomolybdic acid solution and magnetite are mixed, stirred for 12h, wherein, the mass ratio of the dosage of magnetite and phosphomolybdic acid is 2:3;
[0042] (2) The mixed solution obtained in step (1) is subjected to solid-liquid separation, the solid obtained by solid-liquid separation is washed and dried, and then the solid is roasted at 500°C for 3 hours to obtain MoO 3@γ-Fe 2 o 3 Adsorbent;
[0043] (3) get the MoO that 250mg step (2) prepares 3 @γ-Fe 2 o 3 The adsorbent is placed in a fixed-bed reactor, and hydrogen sulfide is passed through at 350°C for 1 hour (flow rate 500mL / min) to obtain MoS x @γ-Fe 2 o 3 Adsorbent, labeled as Adsorbent A.
Embodiment 2
[0044] Example 2 sulfurized γ-Fe 2 o 3 Sorbent preparation
[0045] Take 250mg of gamma-Fe 2 o 3 Place it in a fixed bed reactor and treat it with hydrogen sulfide gas at 350°C for 1 hour to obtain sulfide γ-Fe 2 o 3 Sorbent, labeled Sorbent B.
Embodiment 3
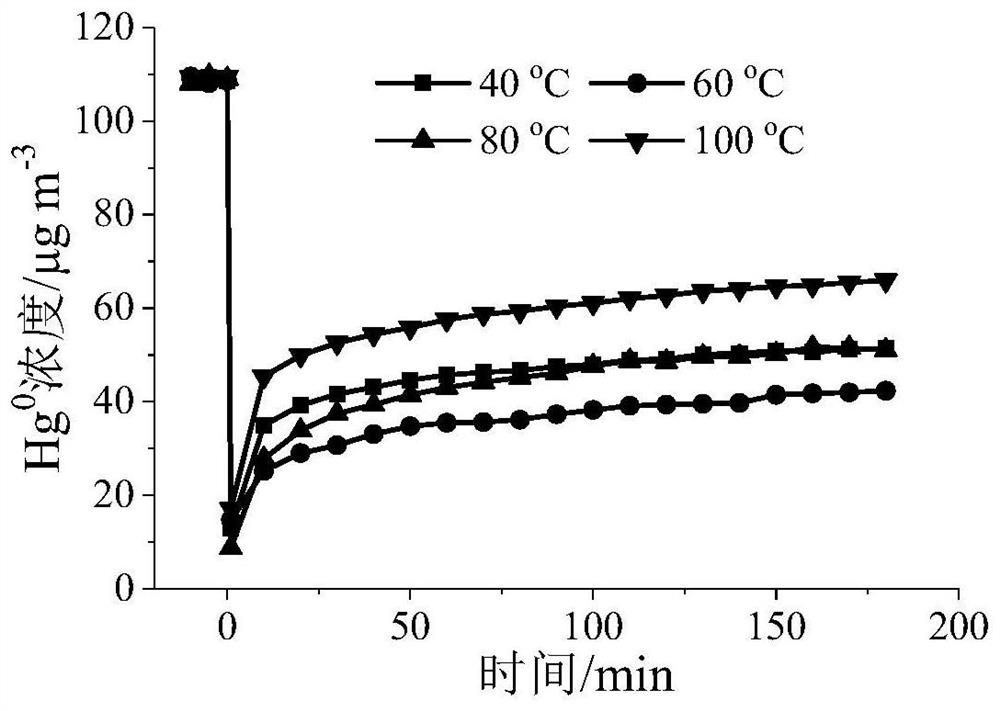
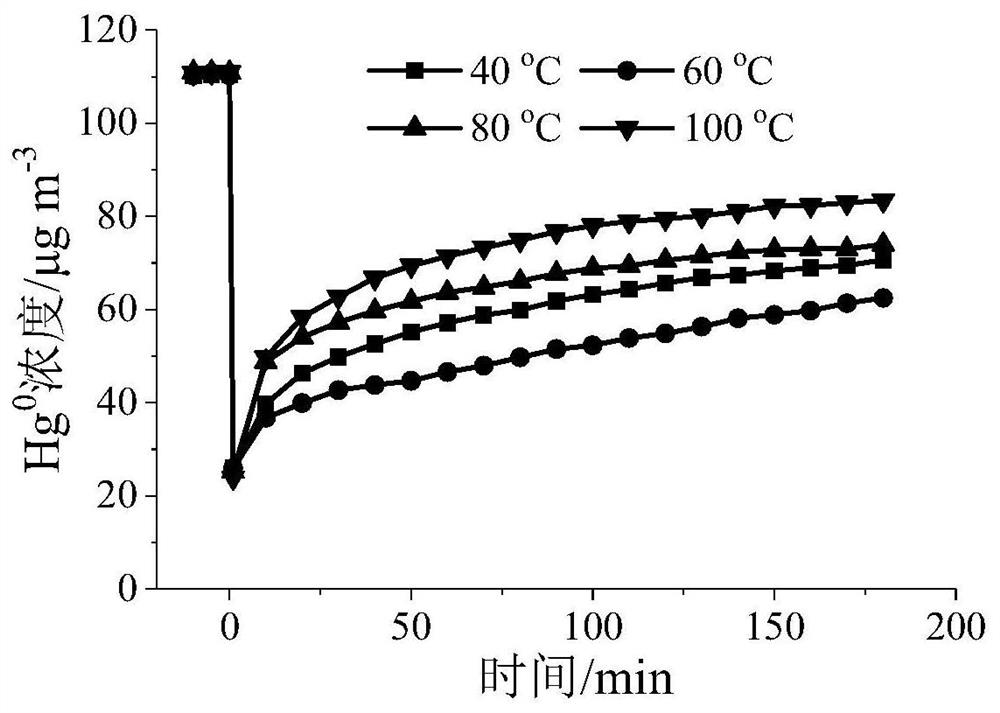
[0046] Example 3 Adsorption of gaseous zero-valent mercury
[0047] Grind and sieve the adsorbents A and B prepared in Example 1 and Example 2, and take 40-60 mesh particles to evaluate the adsorption performance of gaseous zero-valent mercury on a fixed-bed reactor: the amount of adsorbents A and B Both are 20mg, the simulated flue gas composition is: [Hg 0 ] = 110 μg m -3 , nitrogen flow = 500mL min -1 , airspeed=1.5×10 6 cm 3 g -1 h -1 , reaction temperature = 40 ~ 100 ° C, adsorption time = 3h.
[0048] Determination of reactor outlet Hg 0 concentration, and calculate the Hg 0 The adsorption capacity and penetration rate are shown in Table 1.
[0049] Table 1 Hg of different samples 0 Adsorption amount (μg) and penetration rate
[0050]
[0051] It can be seen from Table 1 that under the same reaction conditions, the adsorption of adsorbent A to Hg 0 The adsorption performance of is significantly better than that of adsorbent B. Hg of adsorbent A at 60°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com