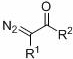
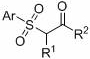
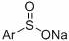A kind of method for preparing β-carbonyl sulfone
A carbonyl sulfone and carbonyl technology, which is applied in the field of preparation of β-carbonyl sulfone, can solve the problems of cumbersome reaction operation, cumbersome reaction steps, and unfriendly environment, and achieve simple reaction operation, good atom economy, and good substrate universality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Add AgNO once in the test tube 3 (0.05 mmol, 8.5 mg), 1,10-phen (0.05 mmol, 9.0 mg), K 2 S 2 o 8 (0.25 mmol, 67.6 mg), 1a (0.5 mmol, 82.1 mg), 2a (1.0 mmol, 114.1 mg), MeCN / H 2 O = 10:1 (2.0 mL). Then the system was heated and stirred at 70°C in air for 4 hours, the reaction system was quenched with saturated sodium sulfite solution, extracted with ethyl acetate three times, and the organic layers were combined and dried with anhydrous sodium sulfate. Then remove the solvent, absorb on silica gel and pass column chromatography to get the product β-carbonyl sulfone 3aa with a yield of 80%. When the reaction was scaled up to 10 mmol (compound 1a), the target product 3aa was still obtained in 74% yield. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0037] 1 H NMR (400 MHz, CDCl 3 ) δ 7.97-7.95 (m, 2H), 7.72-7.68 (m, 1H), 7.6...
Embodiment 2
[0039]
[0040] Add AgNO once in the test tube 3(0.05 mmol, 8.5 mg), 1,10-phen (0.05 mmol, 9.0 mg), K 2 S 2 o 8 (0.25 mmol, 67.6 mg), 1a (0.5 mmol, 82.1 mg), 2b (1.0 mmol, 128.1 mg), MeCN / H 2 O = 10:1 (2.0 mL). Then the system was heated and stirred at 70°C in air for 4 hours, the reaction system was quenched with saturated sodium sulfite solution, extracted with ethyl acetate three times, and the organic layers were combined and dried with anhydrous sodium sulfate. Then the solvent was removed, silica gel was adsorbed, and the product β-carbonyl sulfone 3ab was obtained by column chromatography with a yield of 76%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0041] 1 H NMR (400 MHz, CDCl 3 ) δ 7.97-7.94 (m, 2H), 7.71-7.67 (m, 1H), 7.60-7.57 (m, 2H), 4.99-4.93 (m, 1H), 4.12 (s, 2H), 1.15 (d, J = 6.3 Hz, 6H). 13 CNMR (100 MHz, CDCl 3 ...
Embodiment 3
[0043]
[0044] Add AgNO once in the test tube 3 (0.05 mmol, 8.5 mg), 1,10-phen (0.05 mmol, 9.0 mg), K 2 S 2 o 8 (0.25 mmol, 67.6 mg), 1a (0.5 mmol, 82.1 mg), 2c (1.0 mmol, 142.2 mg), MeCN / H 2 O = 10:1 (2.0 mL). Then the system was heated and stirred at 70°C in air for 4 hours, the reaction system was quenched with saturated sodium sulfite solution, extracted with ethyl acetate three times, and the organic layers were combined and dried with anhydrous sodium sulfate. Then the solvent was removed, silica gel was adsorbed, and the product β-carbonyl sulfone 3ac was obtained by column chromatography with a yield of 70%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0045] 1 H NMR (400 MHz, CDCl 3 ) δ 7.96-7.94 (m, 2H), 7.71-7.67 (m, 1H), 7.60-7.56 (m, 2H), 4.15 (s, 2H), 4.07 (t, J = 6.7 Hz, 2H), 1.55-1.48 (m, 2H),1.31-1.22 (m, 2H), 0.87 (t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com