Pyridone carboxylic acid derivative, preparation method and composition thereof
A composition and hydrate technology, applied in organic chemistry methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of unstable crystal form, excessive solvent residue, poor stability of sitafloxacin derivatives, etc. , to achieve the effect of stable humidity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 recrystallization solvent screening
[0051] In ethanol / ethyl acetate / water, ethanol / acetone / water, methanol / water, ethanol / water, isopropanol / water, acetone / water solvent system, add fumaric acid and sitafloxacin 1.5 hydrate The reaction was heated to make the system basically clear, and then the reaction was stirred for 1 to 2 hours, the temperature was lowered and the crystallization was stirred, the solid was collected by suction filtration, washed with n-heptane, dried in vacuum, and the obtained solid was tested. The results are shown in Table 1.
[0052] Table 1 Screening of recrystallization solvent
[0053]
[0054]
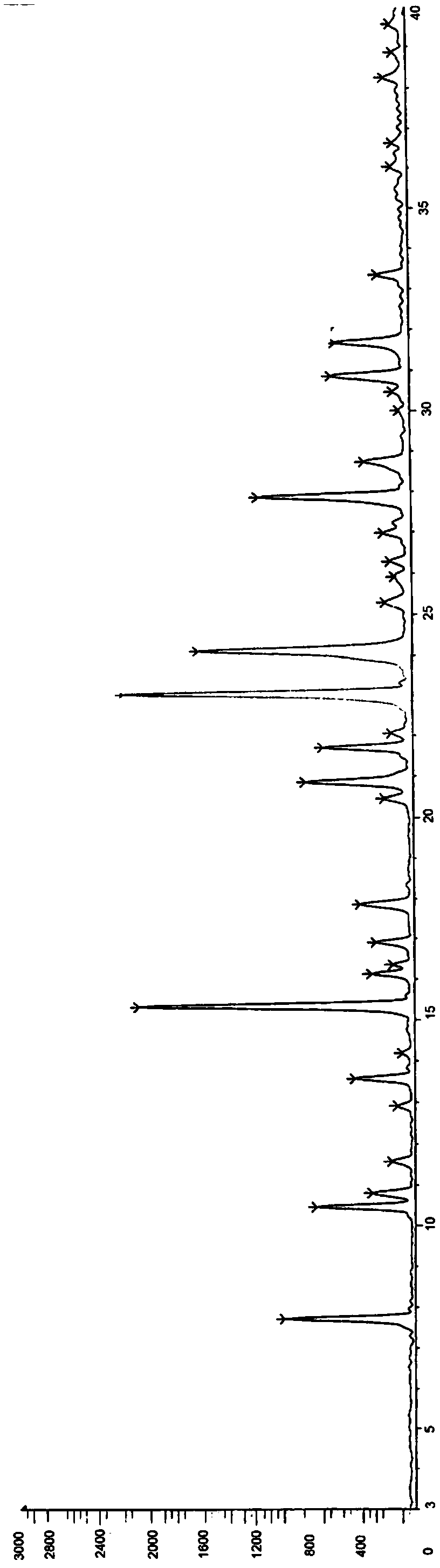
[0055] As can be seen from Table 1, the mixed solvent system of ethanol and water can obtain a single crystal form (alpha crystal form), and the remaining solvent systems can obtain amorphous or mixed crystals. Therefore, it is determined that the mixed solvent of ethanol and water is the recrystallization solvent. The sample o...
Embodiment 2
[0080] Add 10g of sitafloxacin 1.5 hydrate (0.0229mol), 3.2g of fumaric acid (0.0275mol, 1.2equal), 100mL of ethanol and 200mL of water into a 500mL reaction bottle, heat to above 40°C, and stir for 1 to 2 hours , cooled in an ice-water bath, stirred and crystallized for 2 to 5 hours, filtered and washed, and dried in vacuum at 40 to 60°C for 4 to 6 hours to obtain 11.3 g of off-white powdery solid with a yield of 91% and a HPLC purity of 99.95%.
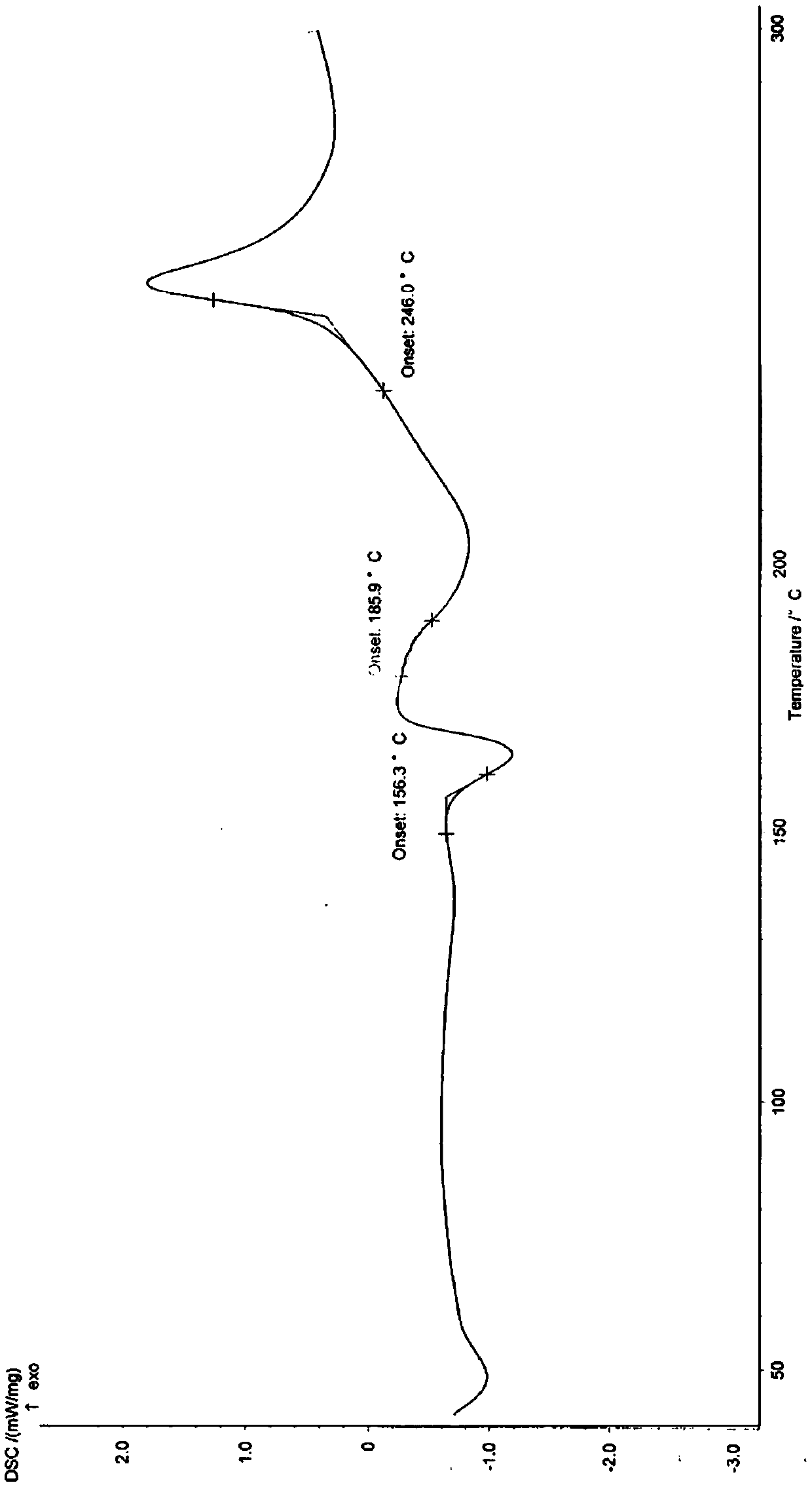
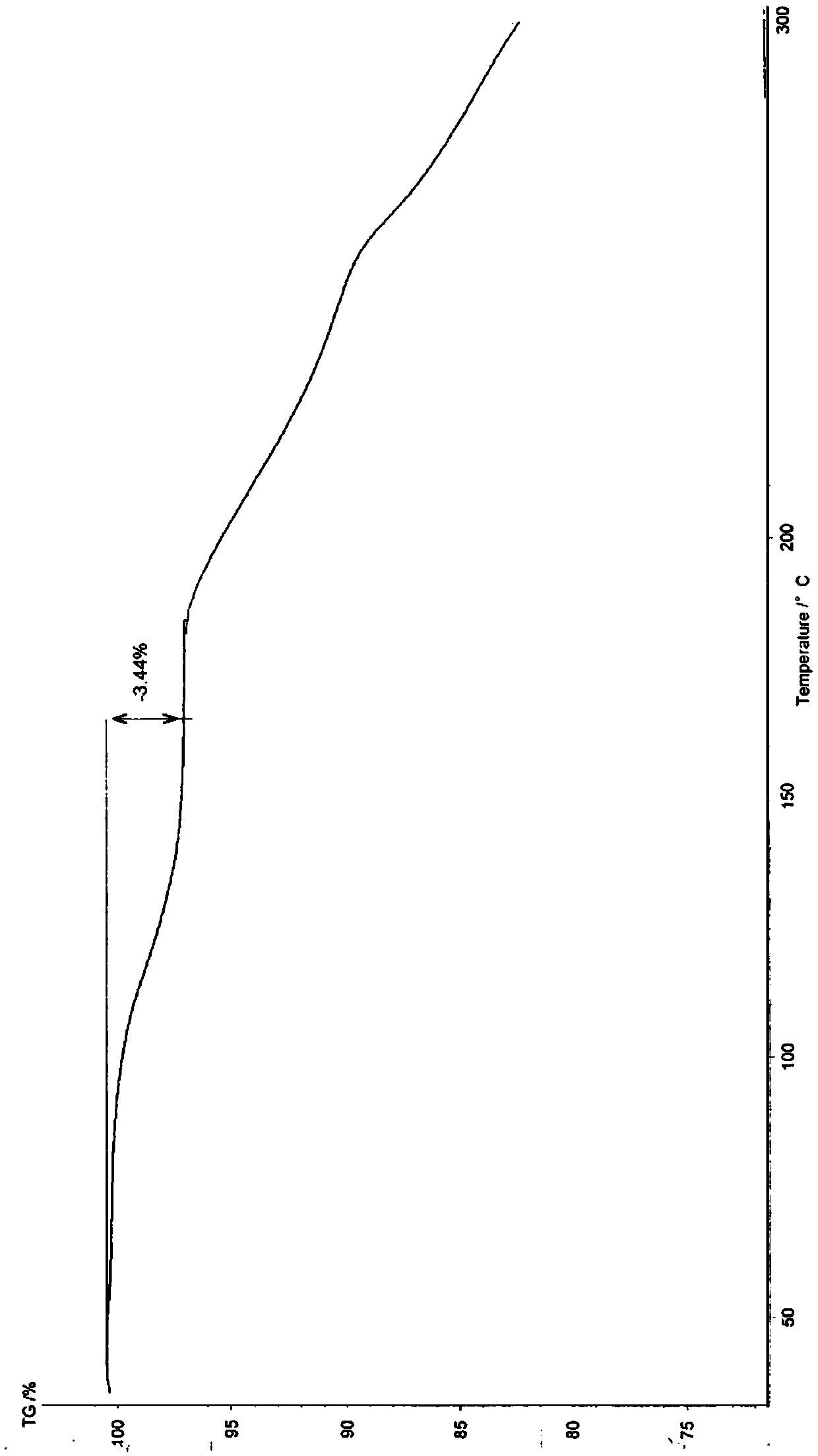
[0081] According to X-ray powder diffraction, X-ray single crystal diffraction, differential thermal analysis and thermogravimetric analysis, the obtained hydrate and crystal form are consistent with the α crystal form in Example 1.
Embodiment 3
[0083] Add 20g of sitafloxacin 1.5 hydrate (0.0458mol), 6.9g of fumaric acid (0.0595mol, 1.3equal), 100mL of ethanol and 500mL of water into a 1L reaction flask, heat to above 40°C, and stir for 1 to 2 hours , cooled in an ice-water bath, stirred and crystallized for 2 to 5 hours, filtered and washed, and dried in vacuum at 40 to 60° C. for 4 to 6 hours to obtain 20.9 g of off-white powdery solid with a yield of 84% and an HPLC purity of 99.96%.
[0084] According to X-ray powder diffraction, X-ray single crystal diffraction, differential thermal analysis and thermogravimetric analysis, the obtained hydrate and crystal form are consistent with the α crystal form in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com