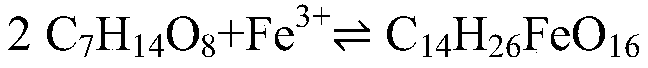Preparation method of iron glucoheptonate
A technology of glucoheptonate and gluconic acid, which is applied in the field of preparation of iron glucoheptonate, can solve the problems of inability to directly obtain high-purity iron glucoheptonate and difficult separation, and achieve simple and environmentally friendly preparation process and high reactivity , the effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
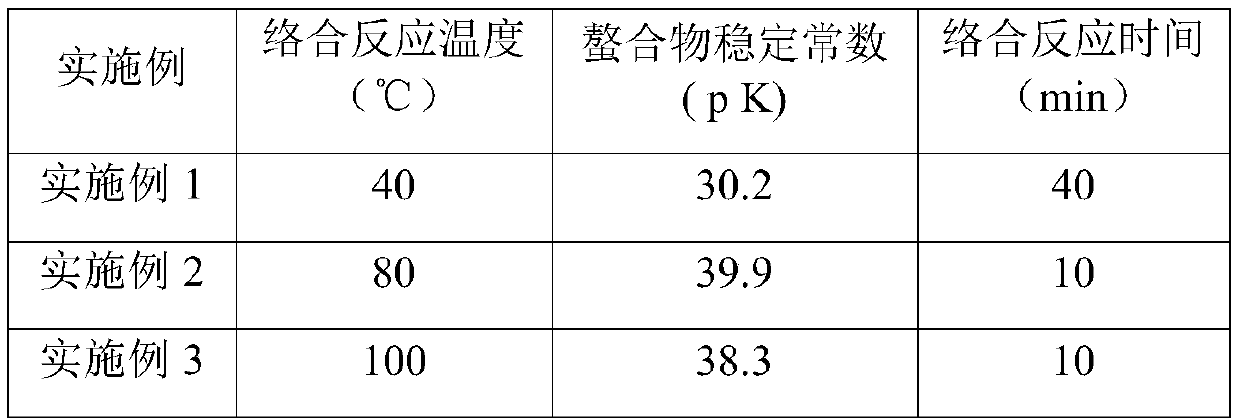
Embodiment 1
[0026] (1) Preparation of gluconic acid aqueous solution:
[0027] Solid sodium glucoheptonate (for example, Wuhan Yuancheng Science and Technology Development Co., Ltd. industrial grade) is formulated into an aqueous solution with a mass percentage of 98%, and then passed through an activated large-pore strong acid cation exchange resin (for example, Shanghai Jinkai Resin Co., Ltd. Company D301) for ion exchange, after sufficient ion exchange, an aqueous solution of glucoheptononic acid with a mass percentage of about 30% is obtained.
[0028] (2) Preparation of ferric hydroxide:
[0029] React 320g of 10% ferrous sulfate aqueous solution with 100g of concentrated ammonia water to form a precipitate, let it stand for stratification, filter the reaction solution, wash the filter cake with deionized water for 2-3 times, blow in air to completely oxidize the filter cake It is reddish-brown powder, which is ferric hydroxide powder.
[0030] (3) Preparation of Iron Glucoheptonat...
Embodiment 2
[0033] (1) Preparation of gluconic acid aqueous solution:
[0034] Solid sodium glucoheptonate (for example, Wuhan Yuancheng Science and Technology Development Co., Ltd. industrial grade) is formulated into an aqueous solution with a mass percentage of 98%, and then passed through an activated large-pore strong acid cation exchange resin (for example, Shanghai Jinkai Resin Co., Ltd. Company D301) for ion exchange, after sufficient ion exchange, an aqueous solution of glucoheptononic acid with a mass percentage of about 30% is obtained.
[0035] (2) Preparation of ferric hydroxide:
[0036] React 320g of 10% ferrous sulfate aqueous solution with 100g of concentrated ammonia water to form a precipitate, let it stand for stratification, filter the reaction solution, wash the filter cake with deionized water for 2-3 times, blow in air to completely oxidize the filter cake to Reddish-brown powder is ferric hydroxide powder.
[0037] (3) Preparation of Iron Glucoheptonate
[0038...
Embodiment 3
[0040] (1) Preparation of gluconic acid aqueous solution:
[0041] Solid sodium glucoheptonate (for example, Wuhan Yuancheng Science and Technology Development Co., Ltd. industrial grade) is formulated into an aqueous solution with a mass percentage of 98%, and then passed through an activated large-pore strong acid cation exchange resin (for example, Shanghai Jinkai Resin Co., Ltd. Company D301) for ion exchange, after sufficient ion exchange, an aqueous solution of glucoheptononic acid with a mass percentage of about 30% is obtained.
[0042] (2) Preparation of ferric hydroxide:
[0043] React 320g of 10% ferrous sulfate aqueous solution with 100g of concentrated ammonia water to form a precipitate, let it stand for stratification, filter the reaction solution, wash the filter cake with deionized water for 2-3 times, blow in air to completely oxidize the filter cake It is reddish-brown powder, which is ferric hydroxide powder.
[0044] (3) Preparation of Iron Glucoheptonat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com