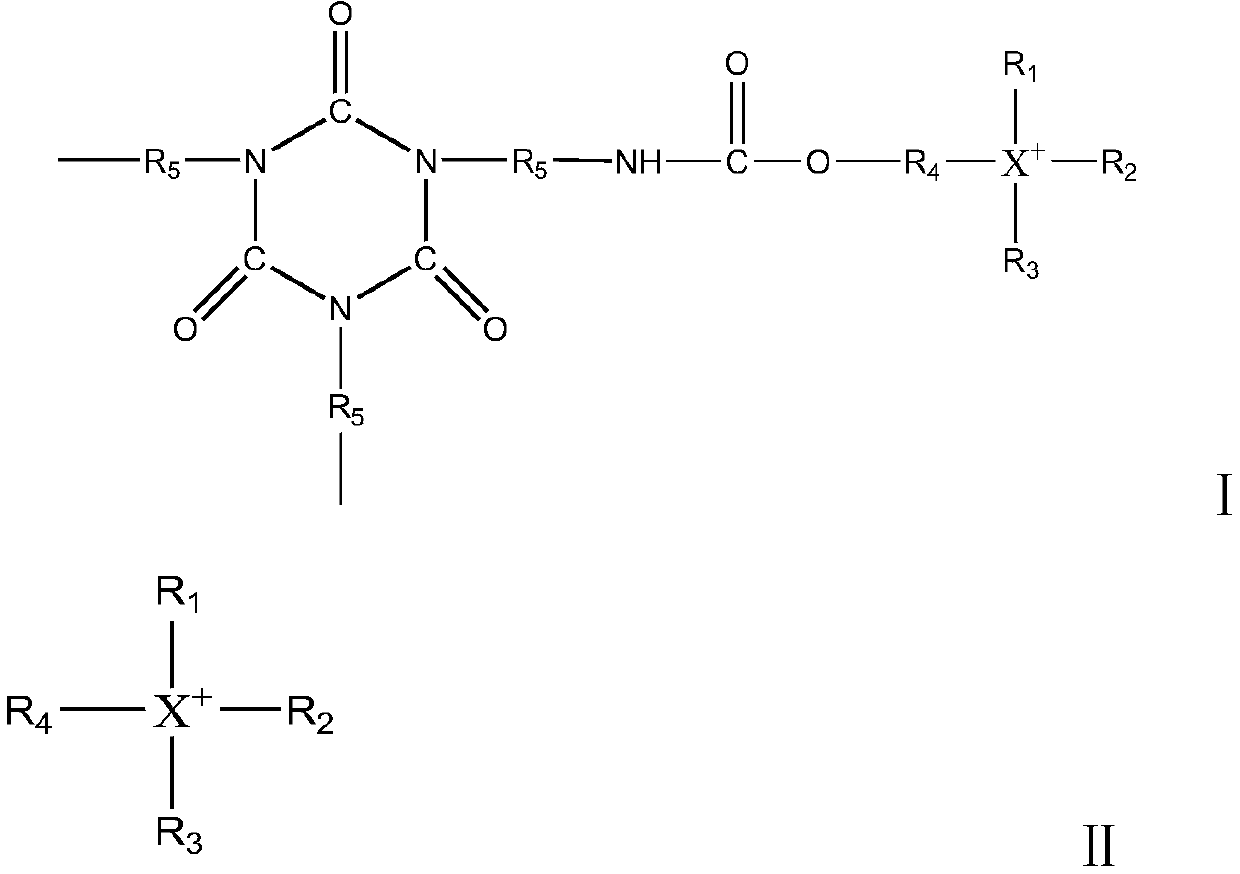
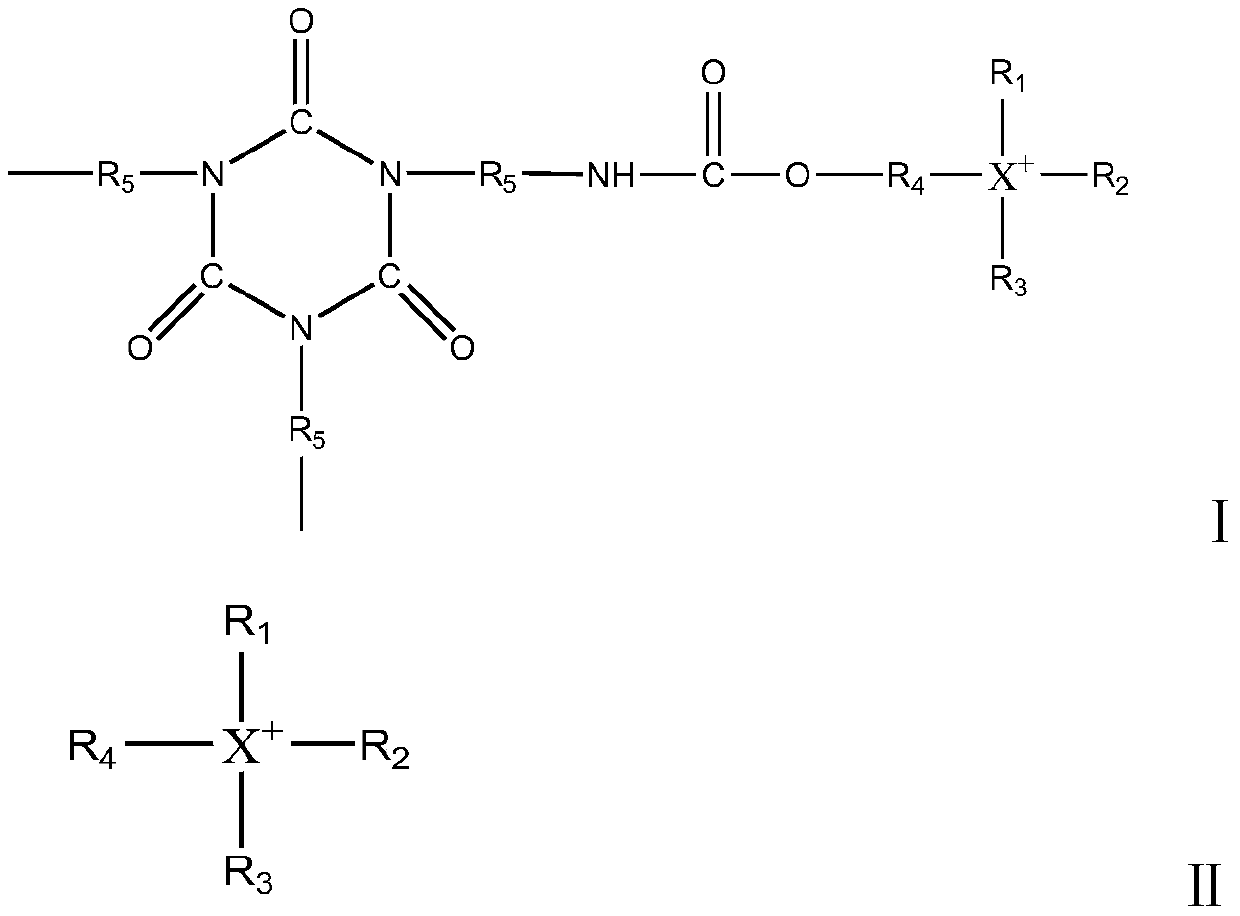
Polyisocyanate composition and preparation method thereof
A technology of polyisocyanate and isocyanate, which is applied in the field of polyisocyanate, can solve problems such as differences in application performance and achieve excellent moisture stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] HDI1000g was heated at 70°C, and 20% by weight of formate of 2-hydroxy-N,N,N-trimethyl-1-propanamine (purchased by Shanghai Qihe Chemical Co., Ltd., CAS No. 62314-25-4) was added. 0.5 g (0.62 mmol) of n-butanol solution, and when the NCO content in the reaction solution reached 39.3% by mass, 0.16 g (0.76 mmol) of di-n-butyl phosphate was added to terminate the reaction. Next, using a thin-film evaporator, it refined twice under the conditions of 130 degreeC and 0.2 Torr, and obtained the polyisocyanate composition whose HDI monomer mass density|concentration was 0.16 mass %. The polyisocyanate composition was heat-treated at 80° C. for 120 minutes to obtain a polyisocyanate composition.
[0064] The chromaticity of the polyisocyanate composition prepared by testing was 27 Hazen, the viscosity was 2930mPa·s (25°C), the NCO content was 21.9% by mass, and the 2-hydroxy-N,N,N-trimethyl-1-propylammonium ion ( The content of compound B) is 0.1 mmol, and the content of polyi...
Embodiment 2
[0066] HDI1000g was heated at 60°C, and 1 g of a 20% by mass n-butanol solution of (3-chloro-2-hydroxypropyl)trimethylammonium chloride (purchased from sigma-aldrich platform, CAS No. 3327-22-8) was added (1.06 mmol), and when the NCO content in the reaction liquid reached 38.9% by mass, 0.41 g (1.27 mmol) of diisooctyl phosphate was added to terminate the reaction. Next, using a thin-film evaporator, it refined twice under the conditions of 130 degreeC and 0.2 Torr, and obtained the polyisocyanate composition whose HDI monomer mass density|concentration was 0.16 mass %. The polyisocyanate composition was heat-treated at 170° C. for 30 minutes to obtain a polyisocyanate composition.
[0067] Various physical properties of the obtained polyisocyanate composition were measured, and the chromaticity was 25 Hazen, the viscosity was 3000 mPa·s (25°C), the NCO content was 21.8% by mass, the HDI monomer mass concentration was 0.14% by mass, (3-chloro -2-hydroxypropyl) trimethylammon...
Embodiment 3
[0069] HDI 1000g was heated at 70°C, and 20 mass% n-butanol solution 2.0g (1.17 mmol), and when the NCO content in the reaction liquid reached 25.4% by mass, 0.179 g (1.4 mmol) of dimethyl sulfate was added to terminate the reaction. Next, using a thin film evaporator, it was refined twice under the conditions of 130° C. and 0.2 Torr to obtain a polyisocyanate composition having a HDI monomer mass concentration of 0.15% by mass. The polyisocyanate composition was heat-treated at 130° C. for 40 minutes to obtain a polyisocyanate composition.
[0070] Various physical properties of the obtained polyisocyanate composition were measured, and the chromaticity was 23 Hazen, the viscosity was 2910 mPa·s (25°C), the NCO content was 22.0% by mass, the mass concentration of HDI monomer was 0.14% by mass, 2-hydroxyethyl The content of base-triphenylphosphonium ion (compound B) is 0.05mmol, and the content of polyisocyanurate derivative (compound A) of 2-hydroxyethyl-triphenylphosphonium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com