Medicine for treating colonitis
A technology for colitis and ulcerative colitis, applied in the field of medicine, can solve problems such as large dosage of drugs, doubtful value of immunosuppressants, obvious side effects, etc., and achieve the effect of enhancing therapeutic effect, overcoming large side effects, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
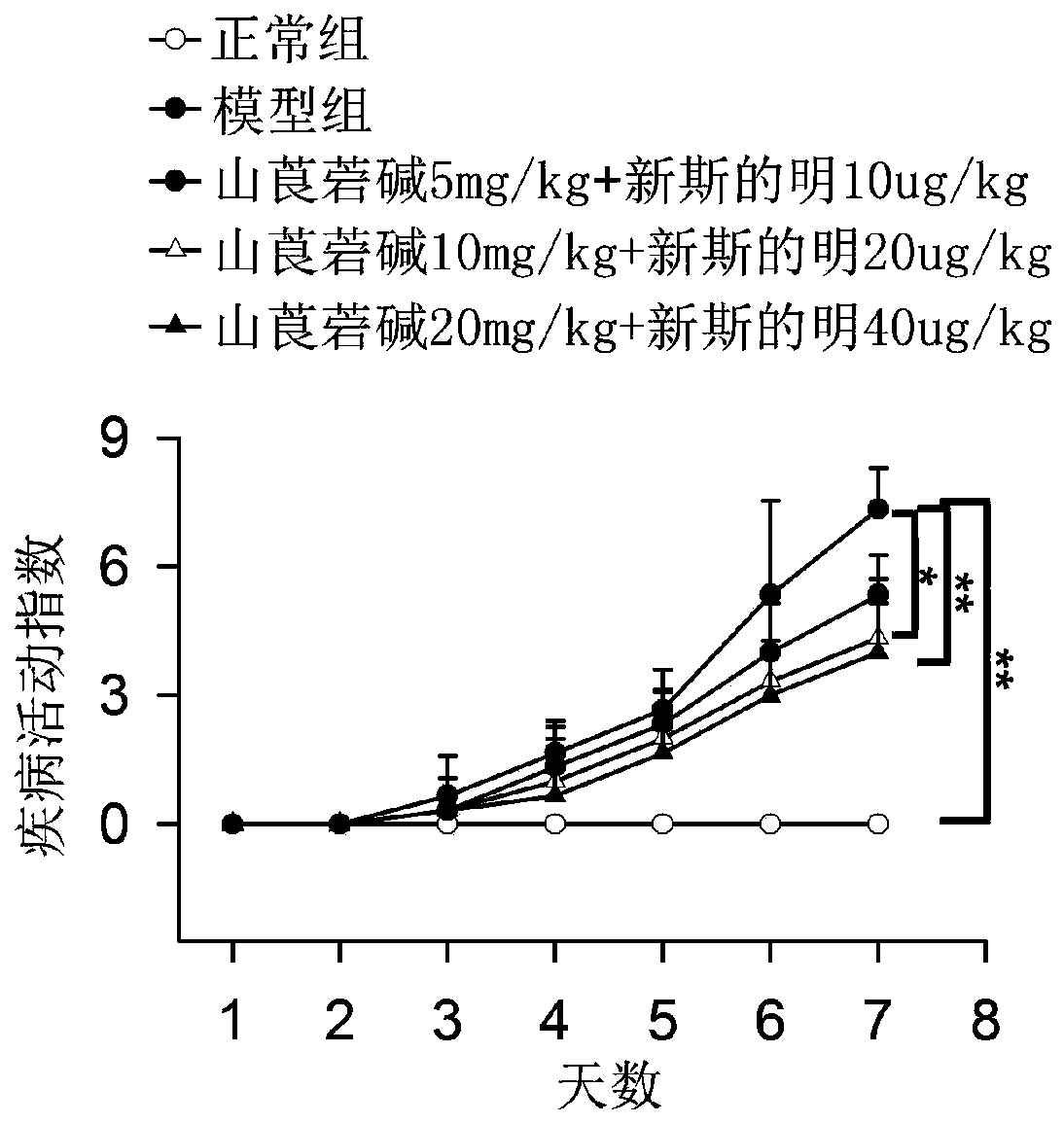
[0026] Example 1 Effect of combined application of racemic anisodamine and neostigmine on disease activity index in mice with ulcerative colitis
[0027] The test drugs racemic anisodamine and neostigmine were divided into three groups (n=10): 5 mg / kg racemic anisodamine and 10 μg / kg neostigmine, 10 mg / kg racemic anisodamine and 20 μg / kg neostigmine, 20 mg / kg racemic anisodamine and 40 μg / kg neostigmine. From the third day of modeling (feeding drinking water containing 3% DSS), racemic anisodamine and neostigmine were administered twice a day. The normal group was given an equal volume of normal saline orally, and drank water normally.
[0028] The model of ulcerative colitis was induced in the mice by feeding water with 3% DSS, and then treated with racemic anisodamine and neostigmine according to the above doses and methods respectively, and then the state of the mice was observed every day.
[0029] The disease activity index (DAI) was assessed as follows: DAI is the sum...
Embodiment 2
[0031] Embodiment 2 Effects of racemic anisodamine and neostigmine combined application on body weight of mice with ulcerative colitis
[0032] Test drugs, mouse model construction, and administration methods are the same as in Example 1.
[0033] The body weight on the first day was taken as 100%, and the change in body weight on this basis was calculated as a percentage.
[0034] The result is as figure 2 As shown, the body weight of mice continued to decrease after DSS modeling, and 10 mg / kg racemic anisodamine and 20 μg / kg neostigmine, 20 mg / kg racemic anisodamine and 40 μg / kg neostigmine could all reduce body weight The degree of decline, of which 20mg / kg racemic anisodamine and 40μg / kg neostigmine effect is better.
Embodiment 3
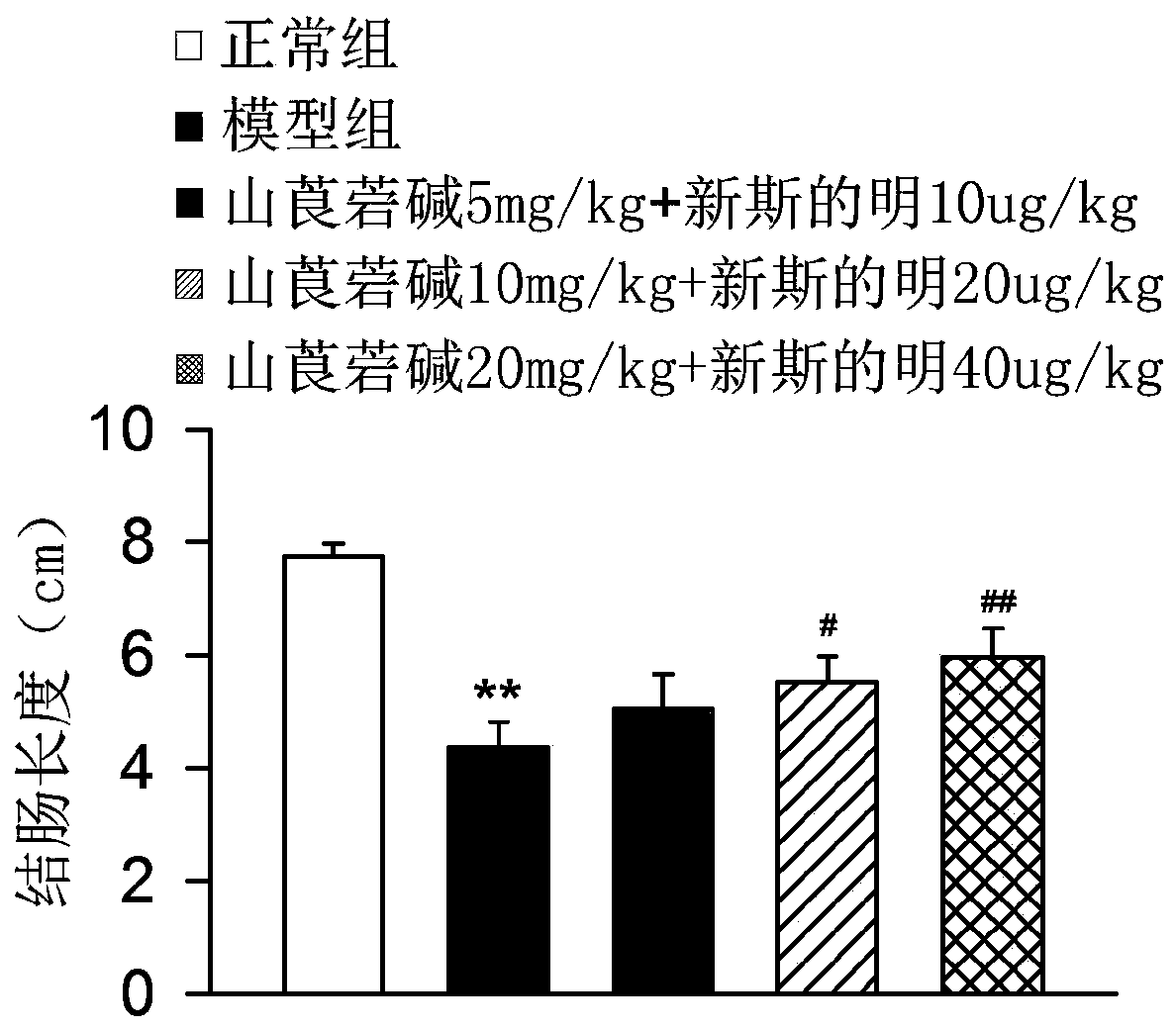
[0035] Example 3 Effect of combined application of racemic anisodamine and neostigmine on colon length in mice with ulcerative colitis
[0036] Test drugs, mouse model construction, and administration methods are the same as in Example 1.
[0037] After 7 days, the mice were sacrificed, and the colons were removed and measured for length.
[0038] The result is as Figure 3-4 As shown, DSS modeling can cause colon inflammation, intestinal wall thinning, and finally lead to shortened colon length, 10mg / kg racemic anisodamine and 20μg / kg neostigmine, 20mg / kg racemic anisodamine and 40μg Neostigmine / kg neostigmine can inhibit the shortening of the colon to varying degrees, among which 20mg / kg racemic anisodamine and 40μg / kg neostigmine have significant effects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com