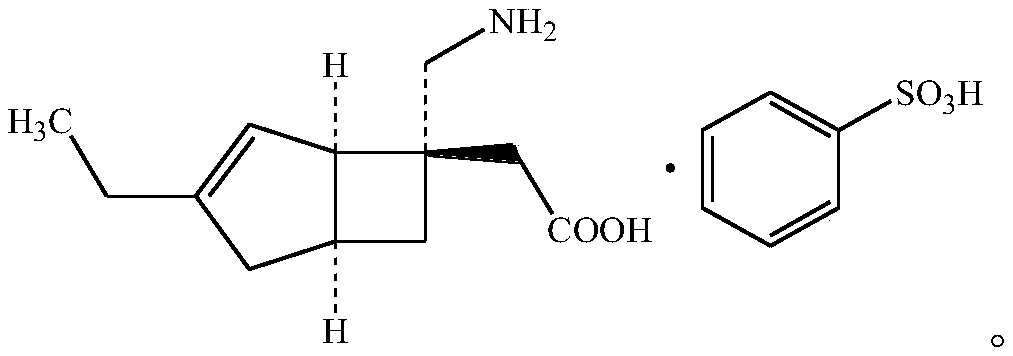
Mirogabalin Besilate orally disintegrating tablet
A technology of orally disintegrating tablets and disintegrating agents, which is applied in the direction of medical preparations of non-active ingredients, pill delivery, metabolic diseases, etc., to achieve the effect of good taste, small stimulation and fast release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
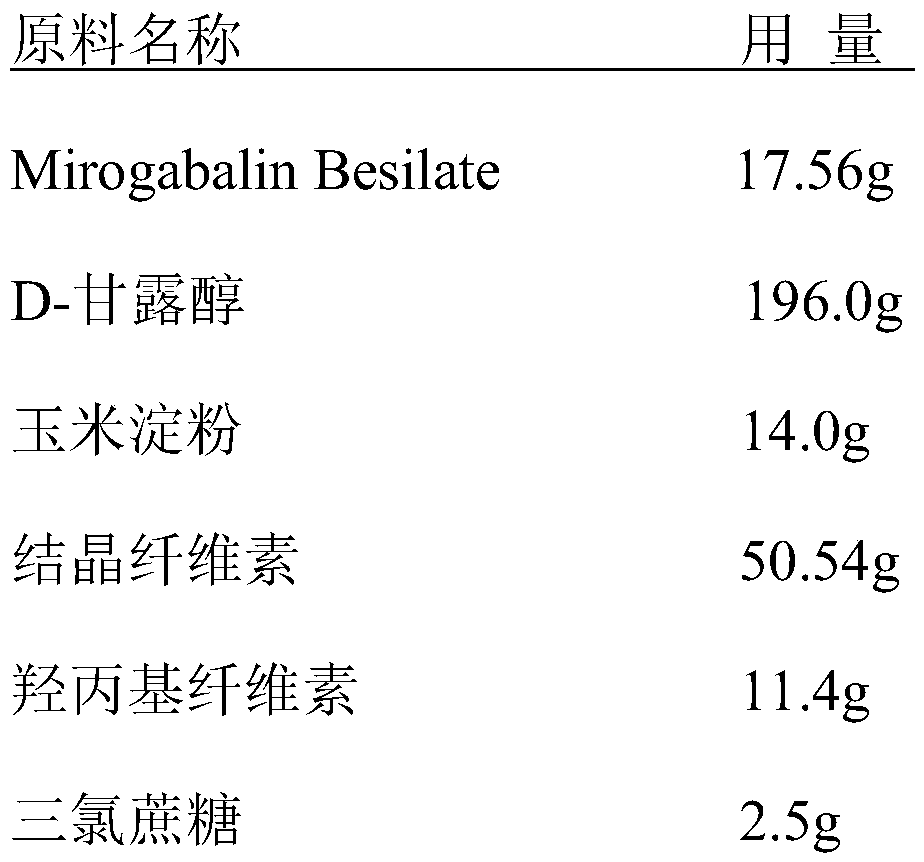
[0036] A prescription for Mirogabalin Besilate orally disintegrating tablets:
[0037]
[0038]
[0039] The preparation method of above-mentioned Mirogabalin Besilate orally disintegrating tablet, comprises the steps:
[0040] According to the D-mannitol of prescription quantity, take half D-mannitol and be mixed with the D-mannitol aqueous solution that mass fraction is 10wt%, in fluidized bed granulator, spray granulation to obtain particle diameter and be 150-250 μm D-mannitol granules;
[0041] Mix Mirogabalin Besilate with D-mannitol granules, corn starch, crystalline cellulose, and the remaining D-mannitol in a column mixer for 5 minutes, add hydroxypropyl cellulose, sucralose, aspartame, L- Menthol and the magnesium stearate mixing of 80wt% prescription quantity, add remaining magnesium stearate mixing again, then flow into the mold of tablet press by the bucket of column mixer under stirring, control environment relative humidity is not More than 60%, compress...
Embodiment 2
[0043] A prescription for Mirogabalin Besilate orally disintegrating tablets:
[0044]
[0045]
[0046] The preparation method of the above-mentioned Mirogabalin Besilate orally disintegrating tablet is the same as that in Example 1, and its specification is 5 mg (in base) / tablet, with a diameter of 7.1 mm, a thickness of 2.6 mm, and a hardness of 30N.
Embodiment 3
[0048] A prescription for Mirogabalin Besilate orally disintegrating tablets:
[0049]
[0050] The preparation method of above-mentioned Mirogabalin Besilate orally disintegrating tablet, comprises the steps:
[0051] According to the D-mannitol of prescription quantity, take half D-mannitol and be mixed with the D-mannitol aqueous solution that mass fraction is 10wt%, in fluidized bed granulator, spray granulation to obtain particle diameter and be 150-250 μm D-mannitol granules;
[0052] Mix Mirogabalin Besilate with D-mannitol granules, crystalline cellulose, and remaining D-mannitol in a column mixer for 5 minutes, add crospovidone, sucralose, acesulfame potassium, L-menthol and 80wt% Mix the prescribed amount of sodium stearyl fumarate, then add the remaining sodium stearyl fumarate and mix evenly, then flow into the mold of the tablet press through the barrel of the column mixer under stirring, and control the relative humidity of the environment The content is not...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com