Viral biological magnetic bead enrichment and concentration kit and its application
A biomagnetic and virus technology, applied in the directions of microorganisms, viruses/phages, biochemical equipment and methods, etc., can solve the problems of requiring centrifugation steps, inconvenient operation, difficult clinical application, etc., achieving simple operation, low cost, avoiding repeated centrifugal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of Virus Biomagnetic Beads Enrichment and Concentration Kit
[0027] The virus biological magnetic bead enrichment and concentration kit provided in this embodiment contains balance solution and Fe 3 o 4 nano magnetic beads.
[0028] The composition of the balance solution is as follows: glucose 200g / L, mannose 100g / L, galactose 50g / L and maltose 50g / L, and the solvent used to prepare the balance solution is 0.9% medical saline.
[0029] Fe 3 o 4 The average particle size of the nano magnetic beads is 1000nm.
Embodiment 2
[0030] The virus enrichment concentration method of embodiment 2 clinical samples
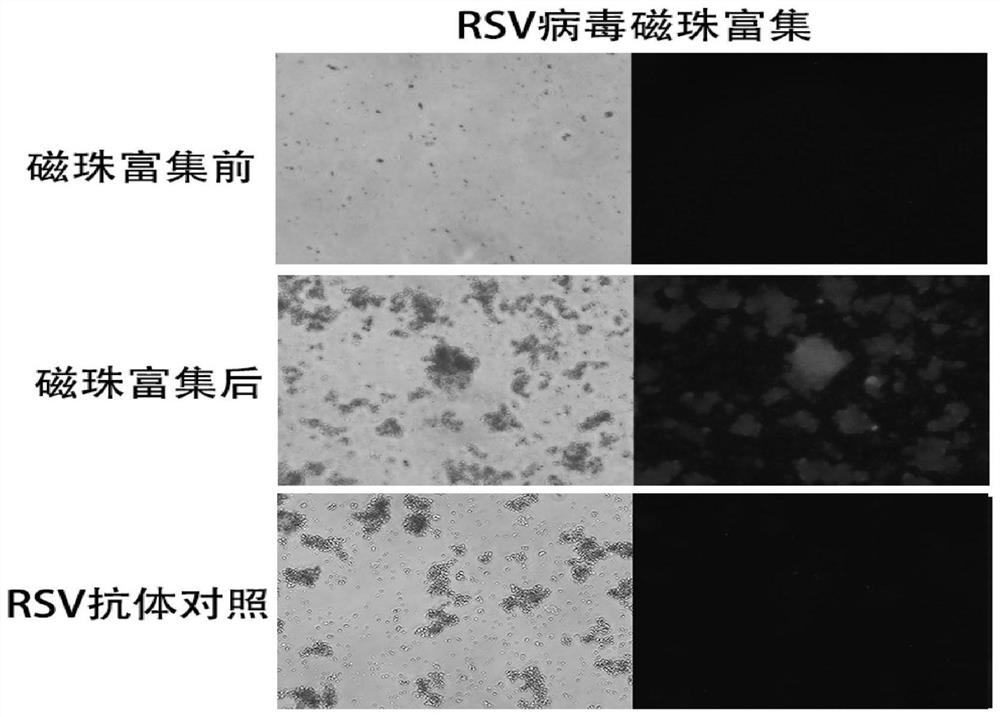
[0031] Take 2ml each of CVB3 and RSV virus liquid respectively (the virus titer is 10 5 PFU / ml), 2 mL of DMEM medium was used for experimental detection.
[0032] Detection process:
[0033] 1. Dissolve 100mg magnetic beads in 100μl balance solution and mix well;
[0034] 2. Carry out testing according to the following groups:
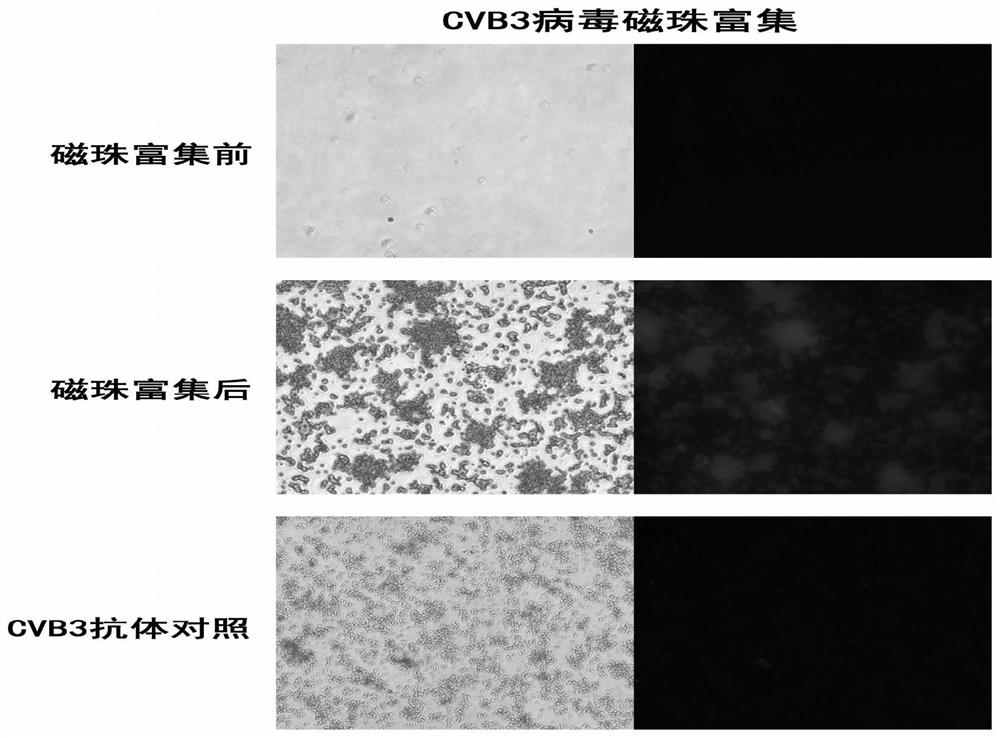
[0035] The first group (before enrichment): 1ml CVB3 virus solution and 100μl primary antibody mouse anti-CVB3 (1:500) were mixed upside down for 20min, then 100μl secondary antibody anti-mouse IgG (1:500) was added and mixed upside down for 20min;
[0036] The second group (experimental group): 1ml CVB3 virus solution and 100μl primary antibody anti-mouse anti-CVB3 (1:500) were mixed upside down for 20 minutes, then 100μl secondary antibody anti-mouse IgG (1:500) was added and mixed upside down for 20 minutes;
[0037] The third group (control group): 1ml DMEM and ...
Embodiment 3
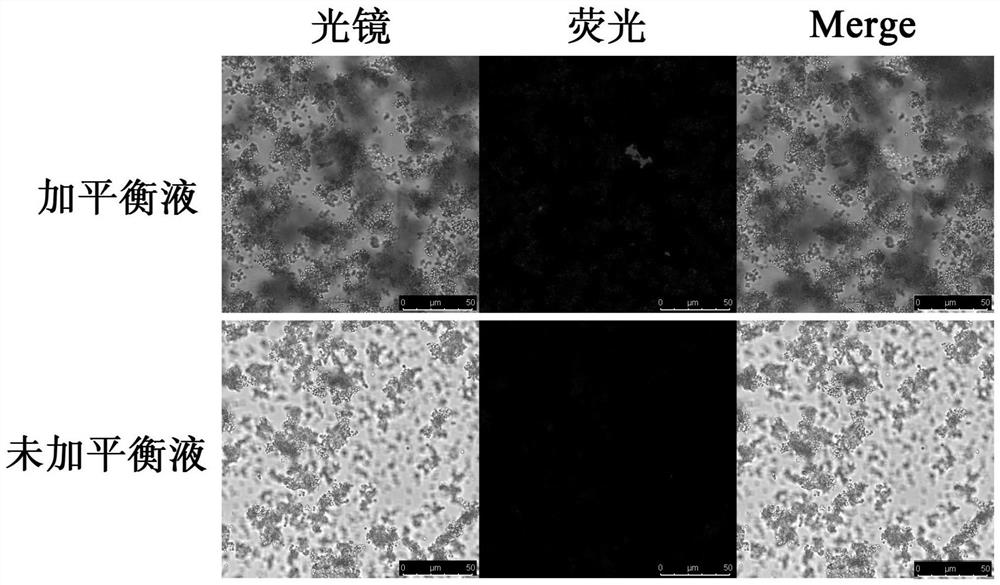
[0048] Embodiment 3 equilibrium liquid stability test
[0049] 1. Thermal stability test
[0050] Place the reagent sample (the balance liquid in Example 1 and the nano-magnetic beads after the balance liquid treatment) in a water bath at 40°C for 3 days in a dark place, no delamination was found by visual observation, and no crystals were precipitated under the microscope, and then the test was carried out according to the above operation steps. The results showed that the enrichment effect was not weakened.
[0051] 2. Cold stability test
[0052]The reagent sample was placed in a -20°C refrigerator out of light for one month, during which it was repeatedly frozen and thawed several times, and then taken out, no stratification was found under the naked eye, and no crystals were precipitated under the microscope. weakened.
[0053] 3. Stability test at room temperature
[0054] The reagent sample was kept at room temperature and protected from light for 1 month. No delami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com