Bisoxazoline ligand compound and synthetic method thereof
A technology of ligand compound and bisoxazoline, which is applied in the field of bisoxazoline ligand compound and its synthesis, can solve the problems of harsh reaction temperature, harsh reaction temperature, lack of etc., and achieve high yield and good enantiometry Body-selective ee value, the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
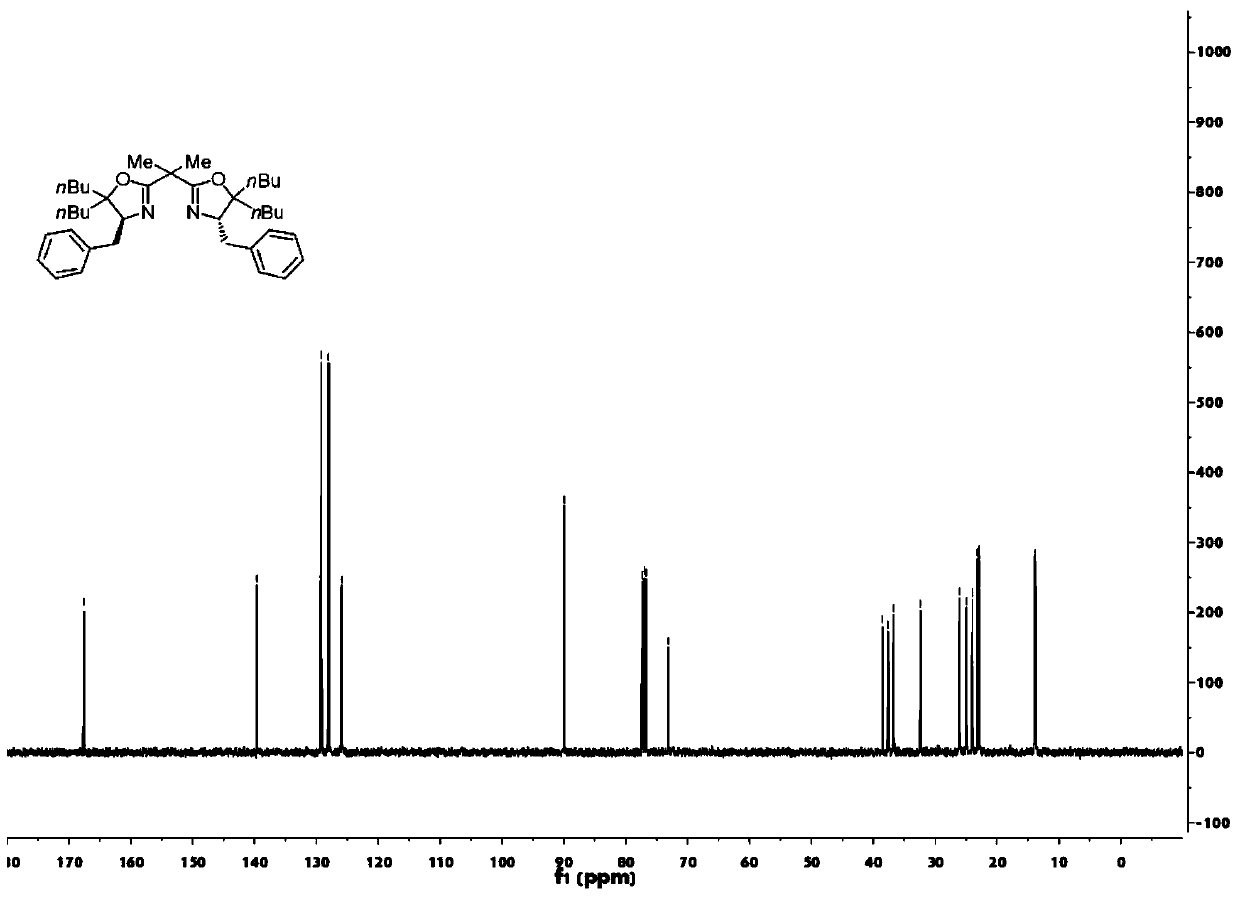
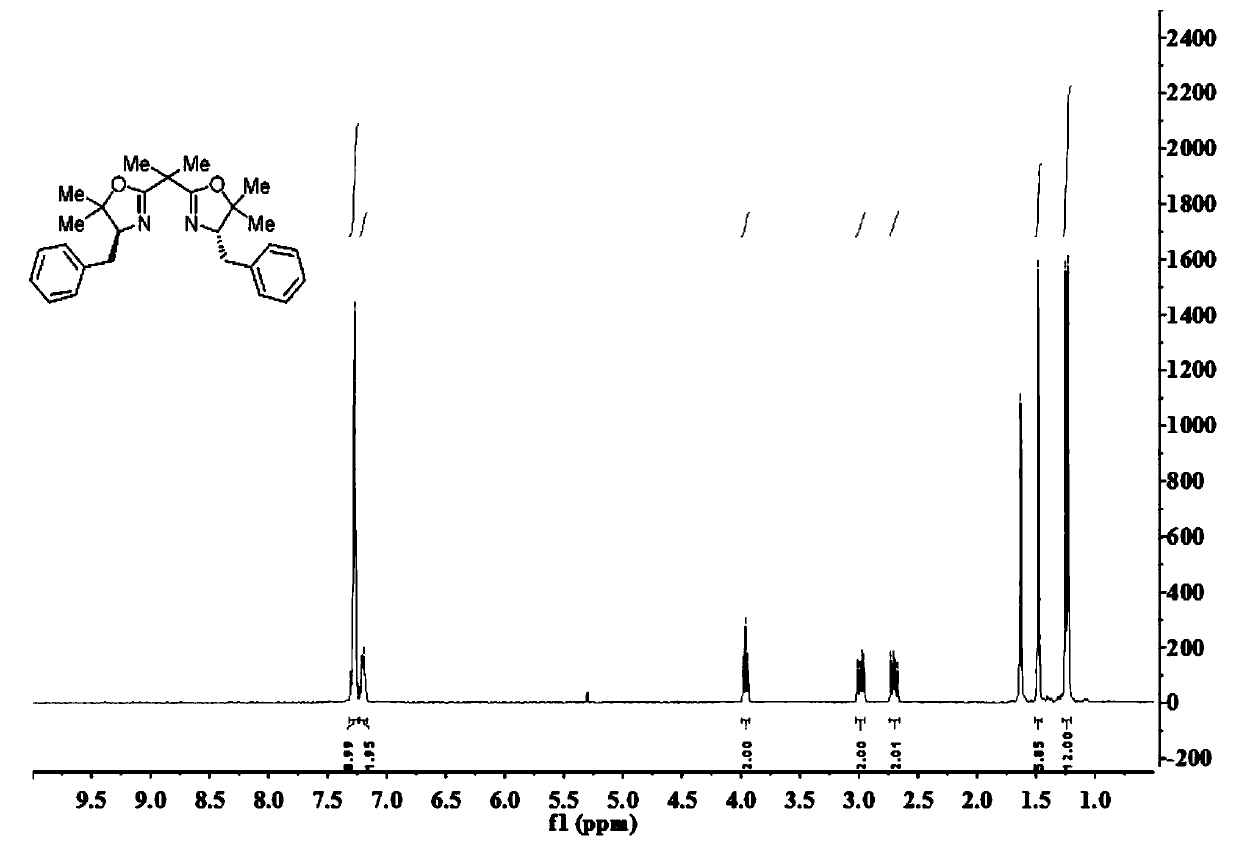
[0049] A kind of bisoxazoline ligand compound
[0050] Its structural formula 7 or 8 is as follows:
[0051]
[0052] Among them, nBu is a n-butyl group, Me is a methyl group, N is a nitrogen atom, and O is an oxygen atom.
Embodiment 2
[0054] A kind of synthetic method of bisoxazoline ligand, comprises the following steps:
[0055] S1), the L-phenylalanine methyl ester hydrochloride 1 of 5g is dissolved in the DCM of 100mL, then add the Et of 8mL 3 N or iPr 2 Net, then add 6mL of Boc dropwise under ice bath 2 O, then transferred to room temperature and stirred for 5 h, monitored by TLC, the reaction of the raw materials was completed, and then adding about 100 mL of 5% citric acid aqueous solution to quench the reaction, extracted with DCM, and then the organic phase was 5% NaHCO 3 (aq.) washing, water washing, anhydrous Na 2 SO 4 Drying, filtering, spin-drying, and passing through the column yielded (tert-butoxycarbonyl)-L-phenylalanine methyl ester 2;
[0056]
[0057] S2), 2.6 g of (tert-butoxycarbonyl)-L-phenylalanine methyl ester 2 prepared in step S1) was dissolved in 10 mL of THF, and then 45 mL of methyl bromide was slowly added dropwise under ice bath or at room temperature Magnesium in tetr...
Embodiment 3
[0066] A kind of synthetic method of bisoxazoline ligand, specifically comprises the following steps:
[0067] S21), the L-phenylalanine methyl ester hydrochloride 1 of 10g was dissolved in the DCM of 200mL, then added the Et of 16.2mL 3 N or iPr 2 Net, then add 13.8mL of Boc dropwise under ice bath 2 O, after being transferred to room temperature and stirred for 5h, TLC monitoring, the raw material reaction was completed, and then adding about 200mL concentration of 5% citric acid aqueous solution to quench the reaction, DCM extraction, and the final organic phase was 5% NaHCO 3 (aq.) washing, water washing, anhydrous Na 2 SO 4 Drying, filtering, spin-drying, and passing through the column yielded (tert-butoxycarbonyl)-L-phenylalanine methyl ester 2;
[0068]
[0069] S22), dissolving 5g of (tert-butoxycarbonyl)-L-phenylalanine methyl ester 2 in 10ml-20ml of THF, then slowly add the freshly prepared n-butyl tetrahydrofuran solution of magnesium bromide, then transferr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com