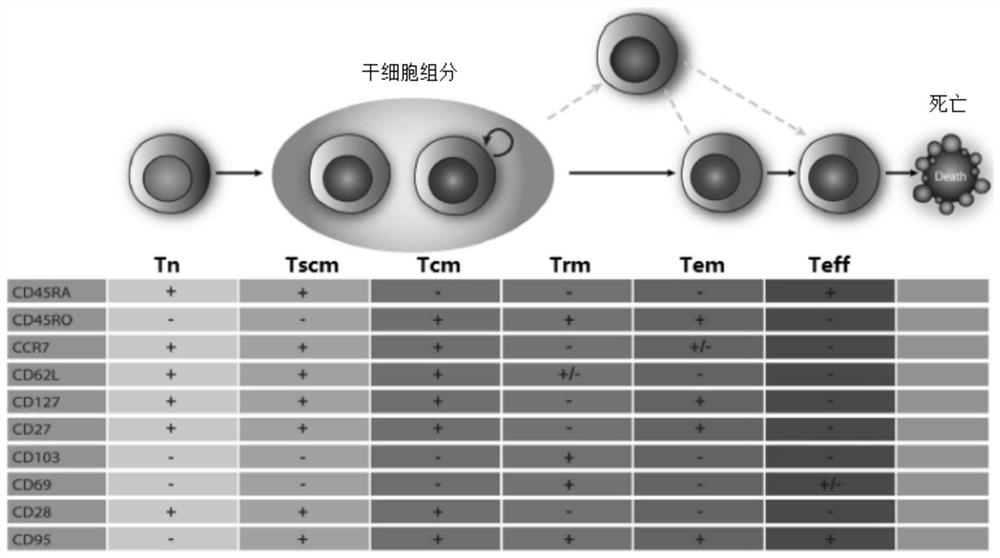
A method for culturing and expanding CD8 T cells
A cell and nuclear cell technology, applied in the field of biomedicine, can solve the problems of unacceptable and difficult patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
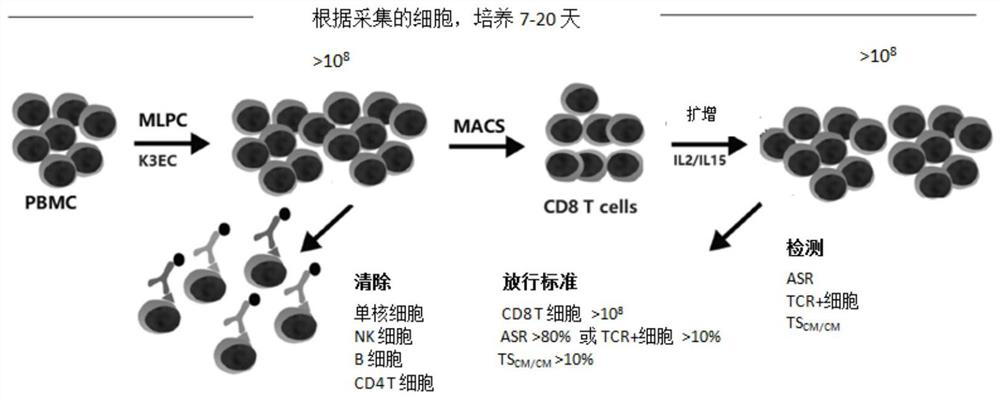
[0066] Embodiment 1 The production process flow of antigen-specific CD8 T cells of the present invention ( figure 2 )
[0067] 1. MLPC-plus activates and expands antigen (CMV-pp65) specific CD8 T cells
[0068] 1) Collect 20ml of peripheral blood, heparin anticoagulation
[0069] 2) PBMCs were collected by Ficoll gradient centrifugation and washed 3 times with PBS
[0070] 3) Resting overnight in 10% FCS-RPMI
[0071] 4) After adding 20% K3EC, centrifuge and discard the supernatant, adjust PBMC to 1x 10 with 3% SG-AIM-V 7 / ml
[0072] 5) Add 1 μg / ml CMV-pp65 antigenic peptide (Miltenyi, Miltenyi, Cat. No. 130-093-435), incubate at 37°C for 2hr
[0073] 6) Supplement 3% SG-AIM-V to 4ml, add 4ml 2x CM, culture and passage in 6-well plate
[0074] 7) Collect cells after 7 days of culture, adjust cells to 1x 10 7 / ml
[0075] 8) Add 1 μg / ml CMV-pp65 antigenic peptide and incubate at 37°C for 2hr
[0076] 9) Fix K3EC cells with paraformaldehyde
[0077] 10) 3% SG-AIM-V...
Embodiment 2
[0086] Example 2 Construction of K562-aAPC (K3EC)
[0087] 1. Basis for the selection of costimulatory molecules
[0088] The main purpose of constructing K3EC is to provide a costimulatory signal for MLPC to activate antigen-specific CD8 T cells. It is currently known that the costimulatory signals required to activate such T cells can be provided by multiple receptors, of which CD28 is the most functional costimulatory receptor. CD28 belongs to the Ig superfamily (IgSF), and its cytoplasmic tail contains three motifs: YMNM, TPRRP and PYAP. When CD28 binds to B7 ligand (CD80 / 86), YMNM activates AP1 (activating protein 1), NF-kB (κB nuclear factor) and NFAT (activating T cell nuclear factor) through PI3K-AKT pathway and Grb2-Vav-Sos pathway ) resulted in Bcl-xL anti-apoptotic protein and IL2 cytokine expression. The PYAP motif can also activate AP1, NF-kB and NFAT transcription factors through the Grb2-Vav-Sos pathway, while the TPRRP motif mainly activates the tyrosine kin...
Embodiment 3
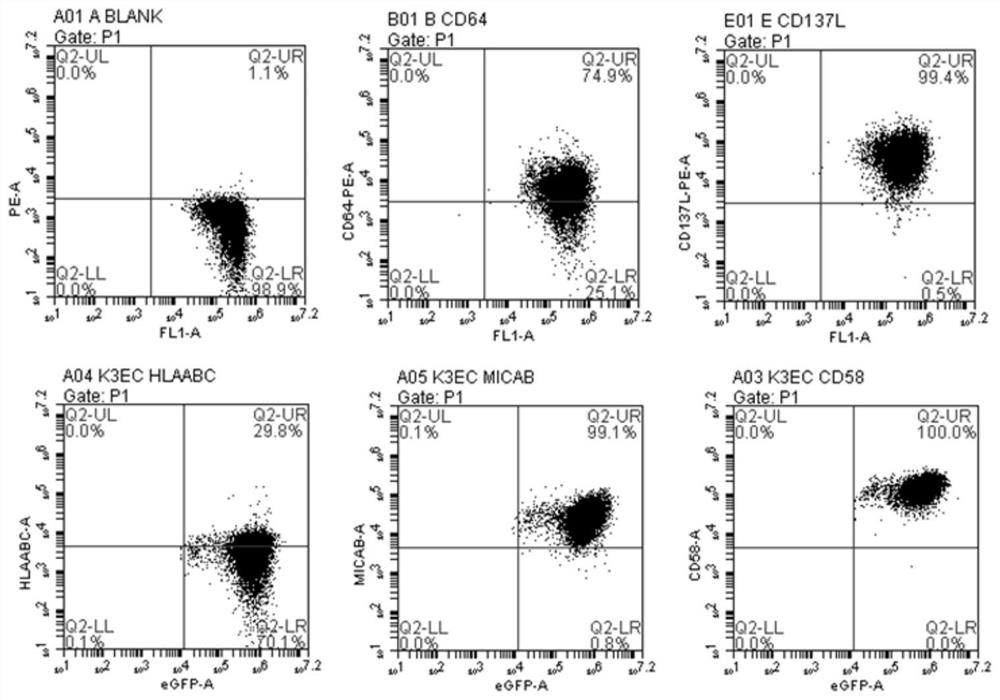
[0107] Example 3 Polyclonal T cell proliferation assay
[0108] aAPC usually needs to be inactivated (ie, the ability to proliferate) before being used for T cell co-culture. This inactivation method includes irradiation, mitomycin treatment, and paraformaldehyde fixation. In order to evaluate whether K3EC still has costimulatory activity after fixation, CD3 antibody was used to provide polyclonal TCR signal, and 4% paraformaldehyde-fixed K3EC provided costimulatory signal. The proliferation activity of T cells was detected by the CFSE dilution method, that is, PBMCs were labeled with CFSE (20 μM). At the same time, a CD3 antibody control group was set up in the plate, and 1 μg / ml CD3 antibody was added to each group. Detection of CD3 after 2 and 5 days of culture + Cells and CD3 + CFSE Low proliferating cells. CD3 + Proliferating cells % = (CD3 + CFSE Low cells / CD3 + cells) x 100%
[0109] Experimental results
[0110] After 2 days of culture, it showed that T ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com