RPA (recombinase polymerase amplification) primer pair, probe, kit and detection method for rapidly detecting Marek's disease virus (MDV)
A Marek's disease and primer pair technology, applied in the field of molecular biology detection, achieves the effects of high sensitivity, strong amplification specificity, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1. Establishment of a Recombinase Polymerase Amplification RPA Method for Rapid Detection of Marek's Disease Virus
[0033] 1. Materials and methods
[0034] 1. Virus Extraction
[0035] According to the instructions of the TIANamp Genomic DNAKit Blood / Cell / Tissue Genomic DNA Extraction Kit from TIANGEN Company, the DNA was extracted from the feather marrow samples of sick chickens. Add 200 µL of virus solution to a 1.5 mL centrifuge tube, add 20 µL of proteinase K and 200 µL of carrier RNA solution, shake the mixture and mix. After 15 min in a 56 °C water bath, add 250 μL of absolute ethanol, transfer to a tube, and centrifuge at 8000 rpm for 1 min. The wash solution was washed twice, then TE was added, left at room temperature for 5 min and centrifuged to obtain DNA / RNA.
[0036] 2. Cloning of target gene
[0037] Meq gene cloning was performed using conventional methods. The meq gene was amplified by conventional PCR. The amplification system was: 95°C / 1...
Embodiment 2
[0054] Embodiment 2. Specificity Test and Sensitivity Test of Recombinase Polymerase Amplification RPA Method for Rapid Detection of Marek's Disease Virus in the Present Invention
[0055] Specificity test: by testing for MDV with positive nucleic acid as well as other nucleic acids from other pathogens, including Newcastle disease virus (NDV), avian infectious anemia virus (CAV), infectious bursal disease virus (IBDV), avian infectivity Bronchitis virus (IBV), infectious laryngotracheitis virus (ILTV), avian influenza virus (AIV) and avian leukosis virus (ALV) and avian reovirus (ARV) were used to determine the specificity of the constructed real-time RPA method. wxya 2 O as a blank control.
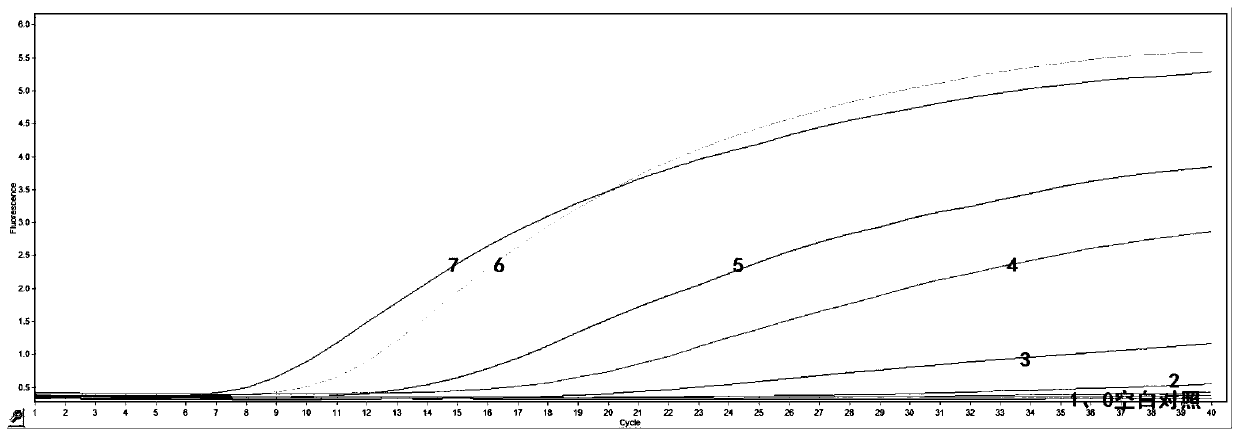
[0056] Specific test results such as figure 2 It was shown that, except for the amplification of MDV, other negative nucleic acids were not amplified, proving that the method of the present invention has good specificity and no cross-reaction with other pathogens.
[0057] Sensitivi...
Embodiment 3
[0059] Embodiment 3, the present invention rapid detection of Marek's disease virus recombinase polymerase amplification RPA method for the detection of clinical samples
[0060] In order to evaluate the practicability of the MDV real-time RPA method constructed in the present invention, 25 clinical samples suspected of MDV infection were detected and compared using the real-time PCR assay. The results showed (Table 2) that the positive detection rate of RPA in 25 clinical samples suspected of MDV infection was 32% (8 / 25), which was consistent with the positive detection rate of real-time PCR. The concordance of positive and negative samples for both methods was 100%.
[0061] Table 2
[0062] Detection method positive sample negative sample The detection rate RPA 8 17 32% PCR 8 17 32%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com