Method for screening tumor neoantigen based on HLA typing and structure
An antigen and tumor technology, applied in the field of tumor neoantigen screening, can solve the problems of high cost, time-consuming, labor-intensive, etc., and achieve the effect of saving labor and funds, reducing the number of experiments, and saving funds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
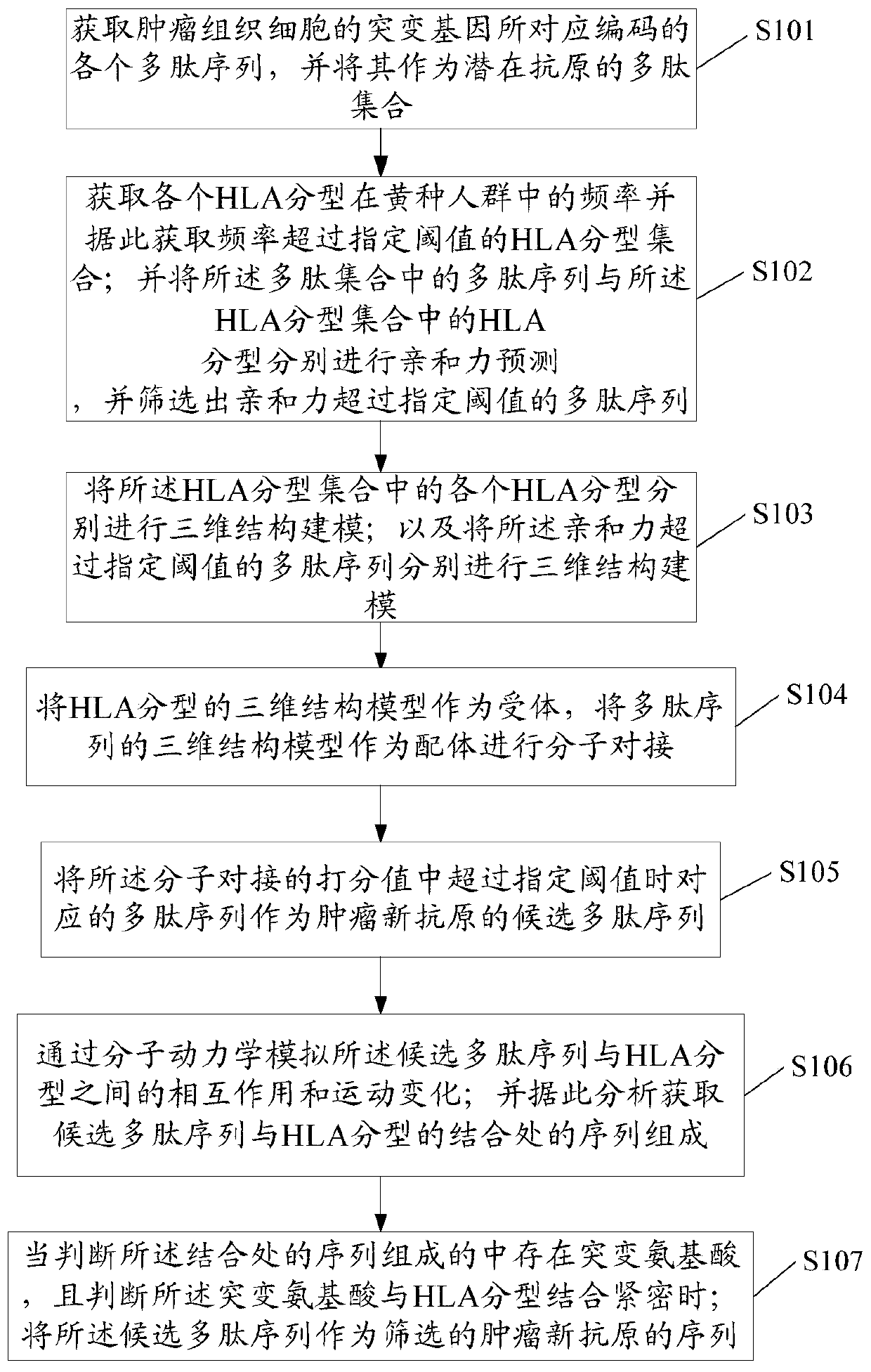
[0050] Such as figure 1 As stated, this embodiment provides a method for screening tumor neoantigens based on HLA typing and structure, including the steps of:
[0051] S101. Obtain the polypeptide sequence encoded by the mutant gene of the patient's tumor tissue cells. Specifically, including:
[0052] A. Extract the DNA of tumor tissue cells by SDS method, and perform DNA sequencing on it;
[0053] B. Comparing the sequenced DNA sequence with the normal wild-type DNA sequence of the tissue cell to obtain a mutated DNA sequence different from the normal wild-type DNA sequence of the tissue cell. Wherein, the normal DNA sequence of the tissue cells may be acquired through an existing database. Wherein, the database may be: COSMIC, NCBI, UCSC, Ensembl, TCGA, etc.
[0054] C. Obtain the polypeptide sequence encoded by the mutated DNA sequence through biological software. Wherein, the biological software can be DNA-man, or other software that can translate DNA sequences into...
Embodiment 4
[0115] Example 4: Evaluation of Neoantigenic Peptide Activity:
[0116] 4.1 T2 cell culture: T2 cells were purchased from ATCC and cultured with 20% FBS IMDM (Gibco) complete medium;
[0117] 4.2 The predicted peptide sequence is synthesized by solid phase, and the purity of the peptide is ≥95%. It is dissolved in DMSO and stored at -80°C;
[0118] 4.3 Add the following raw materials into the 24-well plate: T2 cells, 1X10^6 cells / well; natural human β2 microglobulin (Prospec), the final concentration is 0.5 μM; the final concentration gradient of each polypeptide is set to: 2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM and 80 μM were added to 24-well plates respectively, and co-incubated for 16 hours in a 5% CO2 incubator at 37°C. The experiment set up a blank group and a control group (without adding peptide);
[0119] 4.4 Transfer the cells to a 1.5ml centrifuge tube, wash twice with 1ml 1XPBS, discard the supernatant;
[0120] 4.5 Add FITC Mouse Anti-Human HLA-A2 (BD Biosciences, Ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com