Construction method of pancreatic duct epithelial cell and pancreatic acinar cell co-culture system for simulating in vivo microenvironment
A technology of co-culture system and pancreatic duct, applied in the field of cell biology, can solve problems such as damage, pancreatic mucosal barrier damage, and pancreatic juice spillage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Specific embodiments of the present invention will be described in detail below in conjunction with specific drawings. It should be noted that the technical features or combinations of technical features described in the following embodiments should not be regarded as isolated, and they can be combined with each other to achieve better technical effects.
[0031] The method for establishing the co-culture system of pancreatic ductal epithelial cells and pancreatic acinar cells of the present invention comprises the following steps:
[0032] Pretreatment of the Transwell cell chamber: Coat the Transwell cell chamber with type I collagen (Product No.: C8062, Beijing Suo Laibao Technology Co., Ltd.) for 18-24 hours, wash with PBS and set aside.
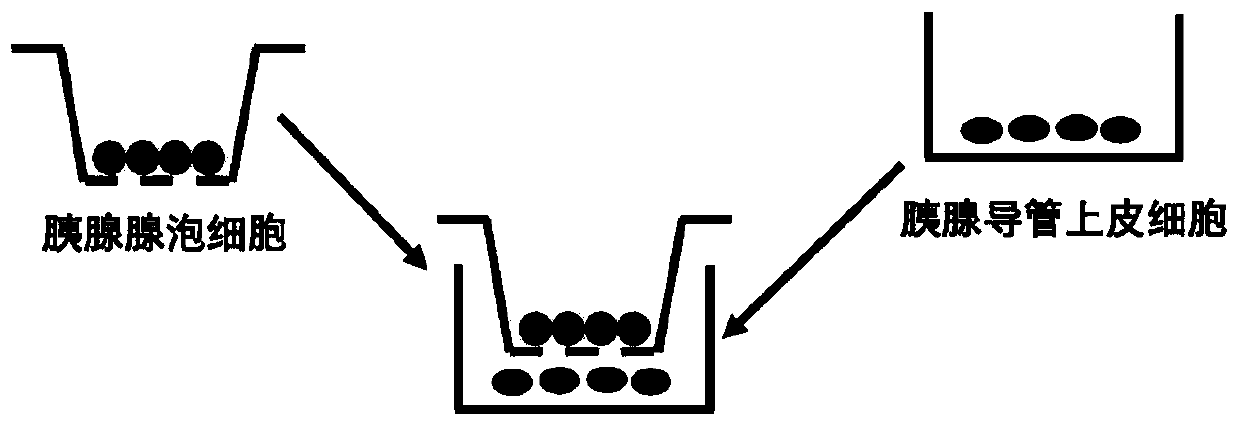
[0033] Extract and isolate primary pancreatic acinar cells from experimental animals; culture HPDE6-C7 human normal pancreatic ductal epithelial cell line; inoculate the resuspended pancreatic acinar cells into the upper layer of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com