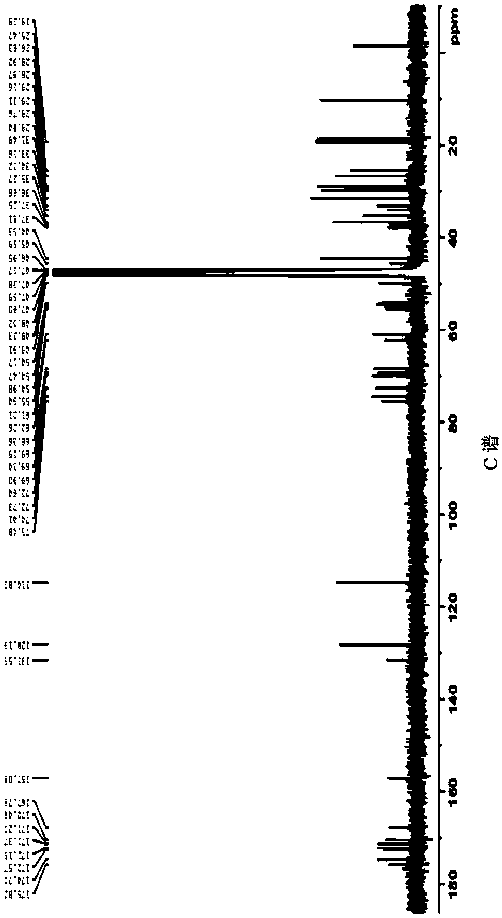
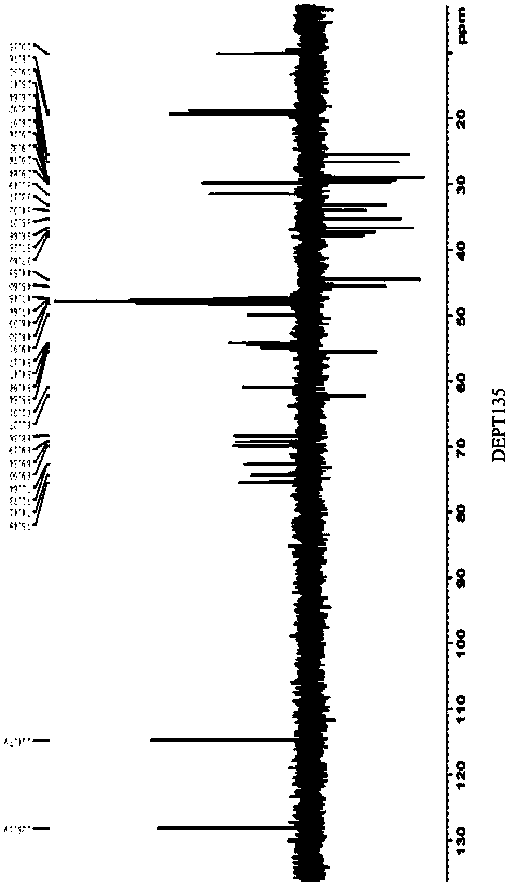
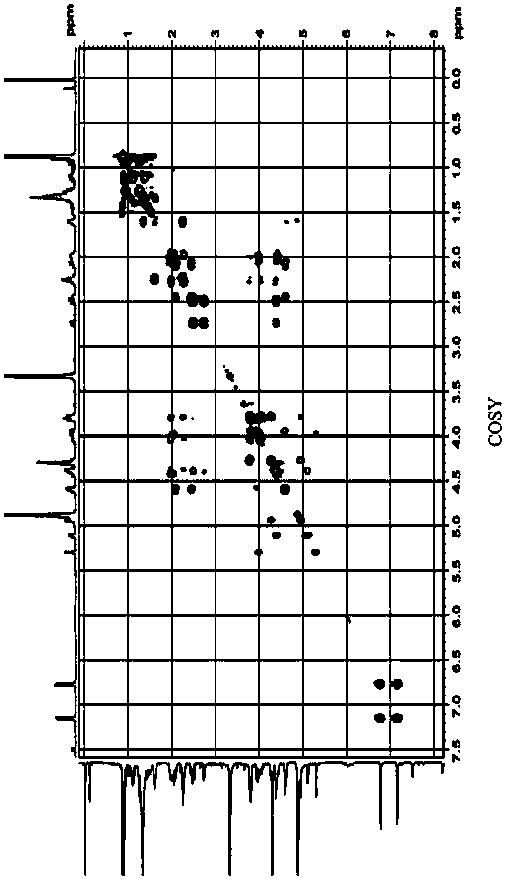
Separation and purification method of pneumocandin B0 serine analog
A technology for separation and purification of pneumocidine, applied in the separation and purification of pneumocidine B0 serine analogues, pneumocidine B0 crude product separation and purification of pneumocidine B0 serine analogues, can solve the problem of not carrying out the separation and preparation of single compounds And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of pneumocantine B0 fermented liquid
[0047] With reference to the Chinese patent No. 201210066411.1 submitted by the applicant on March 14, 2012, the fermented liquid of pneumocidine B0 was prepared. The specific preparation method is as follows.
[0048] Strain: Zalerion arboricola (HCCB30111, CGMCC No.5412);
[0049] Incline medium: PDA;
[0050] Incline culture conditions: 28°C, 5 days;
[0051] Seed Medium (%): Glucose 2.5, KH 2 PO 4 0.9, yeast powder 0.5, drug vehicle 1.0, 85% lactic acid 0.2ml, trace elements 0.1ml, pH 6.0;
[0052] Seed culture conditions: Digging and inoculation, 25°C, 220rpm, 3 days;
[0053] Fermentation medium (%): sucrose 12.5, sodium glutamate 0.8, yeast powder 0.8, proline 1.5, KH 2 PO 4 0.15, magnesium sulfate heptahydrate 0.04, trace elements 1ml, MES buffer salt 1.5, pH 5.3;
[0054] Fermentation conditions: 10% seed transfer, 25°C, 220rpm, 7 days of fermentation;
[0055] Trace element compos...
Embodiment 2
[0057] Embodiment 2: the preparation of the crude product of pneumocidine B0 containing serine analog
[0058] With reference to the Chinese patent No. 201210066411.1 submitted by the applicant on March 14, 2012, the crude product of pneumocidine B0 containing serine analogs was prepared. The specific preparation method is as follows.
[0059] Washing and filtering: adopt ceramic membrane microfiltration equipment, carry out cross-flow filtration to 30L of pneumocantine B0 fermented liquid prepared by embodiment 1, obtain concentrated mycelium suspension; use 10% phosphoric acid in 0.05 μm ceramic membrane Adjust the pH to 3.0 to wash and filter the mycelia suspension.
[0060] Extraction and filtration: After washing with water, filter until about 15L of feed liquid containing mycelia is extracted directly with methanol-water solution (methanol volume ratio is 20%) ceramic membrane, and the temperature is controlled at 10-15°C for extraction and filtration operate.
[0061...
Embodiment 3
[0068] Embodiment 3: Separation and preparation of nemocontin B0 serine analog
[0069] a) The first chromatographic enrichment of nemocontin B0 serine analogs
[0070] The crude product of neomocontin B0 containing serine analogs is enriched by the first chromatographic enrichment with a polymer matrix chromatographic filler, such as UniPS from Navitas, with a particle size of 10 μm and a pore size of 300 A. The mobile phase is aqueous methanol, methanol The ratio is 75% (volume ratio), isocratic elution, and fractional collection to obtain the first chromatographic enrichment solution containing neumeridine B0 serine analogue with a purity > 30%.
[0071] b) The second chromatographic purification of nemocontin B0 serine analog
[0072] Concentrate under reduced pressure to remove the solvent from the first chromatographic enrichment liquid containing nemocontin B0 serine analogs with a purity > 30%, and then use a hydrophilic interaction chromatography filler, such as XAmi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com