a co 2 Preparation method of p/npc electrocatalyst
An electrocatalyst, 2·6H2O technology, applied in the direction of circuits, electrical components, battery electrodes, etc., can solve the problems of high OER overpotential, low electron transfer efficiency, poor dual functionality, etc., achieve good stability and improve oxygen reduction performance , High electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Put 0.1gC 14 H 14 N 3 SO 3 Na was added to 120 mL of ultrapure water, and added in an ice-water bath with stirring.
[0019] Add 0.48g FeCl 3 ·6H 2 O, add 0.2mL C 4 H 5 N; then add 57.3mg (NPC1 2 ) 3 , and kept stirring for 24h in the dark under normal temperature conditions, and the precursor polypyrrole was obtained through suction filtration, washing and drying;
[0020] (2) Put 60 mg of polypyrrole in 0.29 g of Co(NO 3 ) 2 ·6H 2 O ultrapure aqueous solution (10ml)
[0021] Ultrasonic for 20min, let stand for 12h, centrifuge, wash and dry; 4 H 6 N 2 The ultrapure aqueous solution (10ml) was left standing for 12h, centrifuged, washed and dried. The obtained sample was placed in the center of the tube furnace through 900 oC Pyrolysis at high temperature for 1h to obtain Co 2 P / NPC electrocatalysts. In this step, the annealing atmosphere is high-purity Ar, and the heating rate of the tube furnace is 10 o C / min.
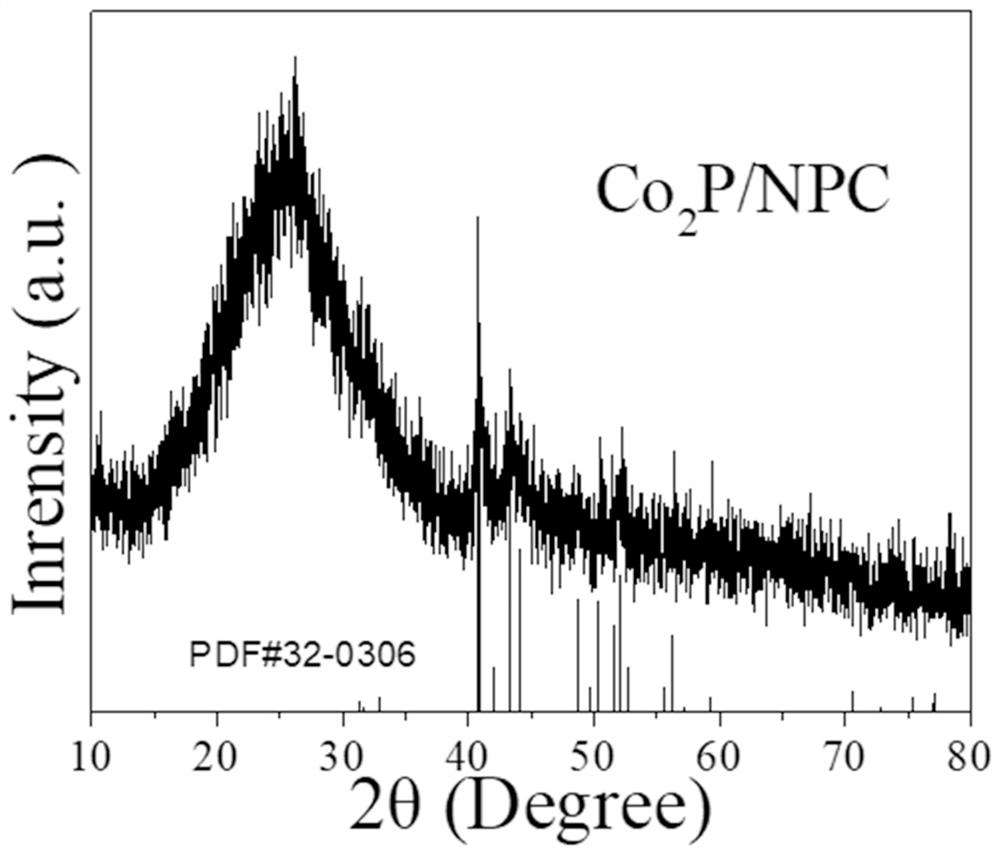
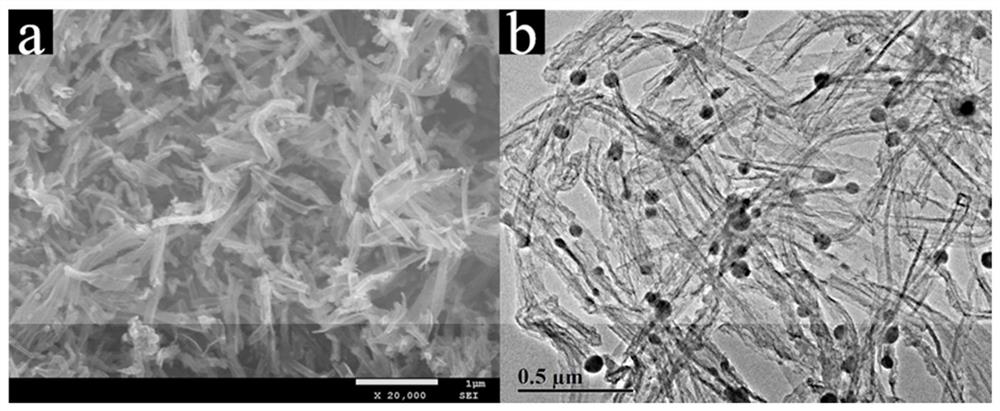
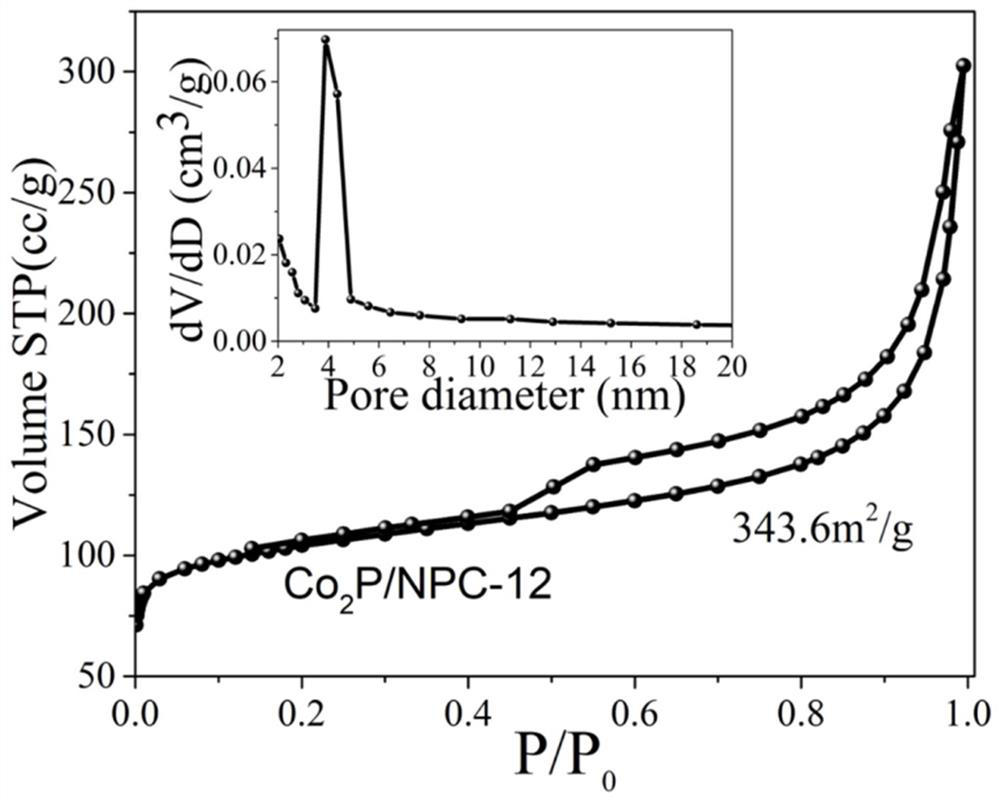
[0022] figure 1 Co prepared und...
Embodiment 2
[0025] (1) Put 0.1gC 14 H 14 N 3 SO 3 Na was added to 120mL ultrapure water, and 0.48g FeCl was added in an ice-water bath stirred environment 3 ·6H 2 O, add 0.2mL C 4 H 5 N; then add 57.3mg (NPC1 2 ) 3, and kept stirring for 24h in the dark under normal temperature conditions, and the precursor polypyrrole was obtained through suction filtration, washing and drying;
[0026] (2) Put 60 mg of polypyrrole in 0.29 g of Co(NO 3 ) 2 ·6H 2 O ultrapure aqueous solution (10ml) was sonicated for 20min, left standing for 5h, centrifuged, washed and dried; the obtained sample was placed in a solution containing 0.082g C 4 H 6 N 2 The ultrapure aqueous solution (10ml) was left standing for 5h, centrifuged, washed and dried. The obtained sample was placed in the center of the tube furnace through 900 oC Pyrolysis at high temperature for 1h to obtain Co 2 P / NPC electrocatalysts. In this step, the annealing atmosphere is high-purity Ar, and the heating rate of the tube furn...
Embodiment 3
[0029] (1) Put 0.1g C 14 H 14 N 3 SO 3 Na was added to 120mL ultrapure water, and 0.48g FeCl was added in an ice-water bath stirred environment 3 ·6H 2 O, add 0.2mL C 4 H 5 N; then add 57.3mg (NPC1 2 ) 3 , and kept stirring for 24h in the dark under normal temperature conditions. After suction filtration, washing and drying, the precursor polypyrrole was obtained; 60mg of polypyrrole was placed in 0.29g of Co(NO) 3 ) 2 ·6H 2 O ultrapure aqueous solution (10ml) was sonicated for 20min, left standing for 2h, centrifuged, washed and dried; the obtained sample was placed in a solution containing 0.082g C 4 H 6 N 2 The ultrapure aqueous solution (10ml) was left standing for 2h, centrifuged, washed and dried. The obtained sample was placed in the center of the tube furnace through 900 oC Pyrolysis at high temperature for 1h to obtain Co 2 P / NPC electrocatalysts. In this step, the annealing atmosphere is high-purity Ar, and the heating rate of the tube furnace is 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com