Oxygen evolution electrocatalytic material, preparation method and applications thereof
A technology of electrocatalytic materials and catalytic materials, applied in the field of oxygen evolution electrocatalytic materials and their preparation, can solve the problems of insufficient exposure of active sites and inability to maximize the use of catalytic activity, so as to promote electron and mass transfer, The effect of accelerating reaction kinetics and high current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Pretreatment of the substrate: 0.8g of stainless steel (SS, 304, containing Fe: 50-60wt%, Ni: 8-11wt%, Cr: 18-20wt%, 1000 mesh) with a size of 4cm × 2.5cm Submerged in dilute hydrochloric acid (2.0mol / L HCl), ethanol and distilled water and ultrasonically washed to remove the surface oxide layer and organic residues, and then dried in an oven at 60°C.
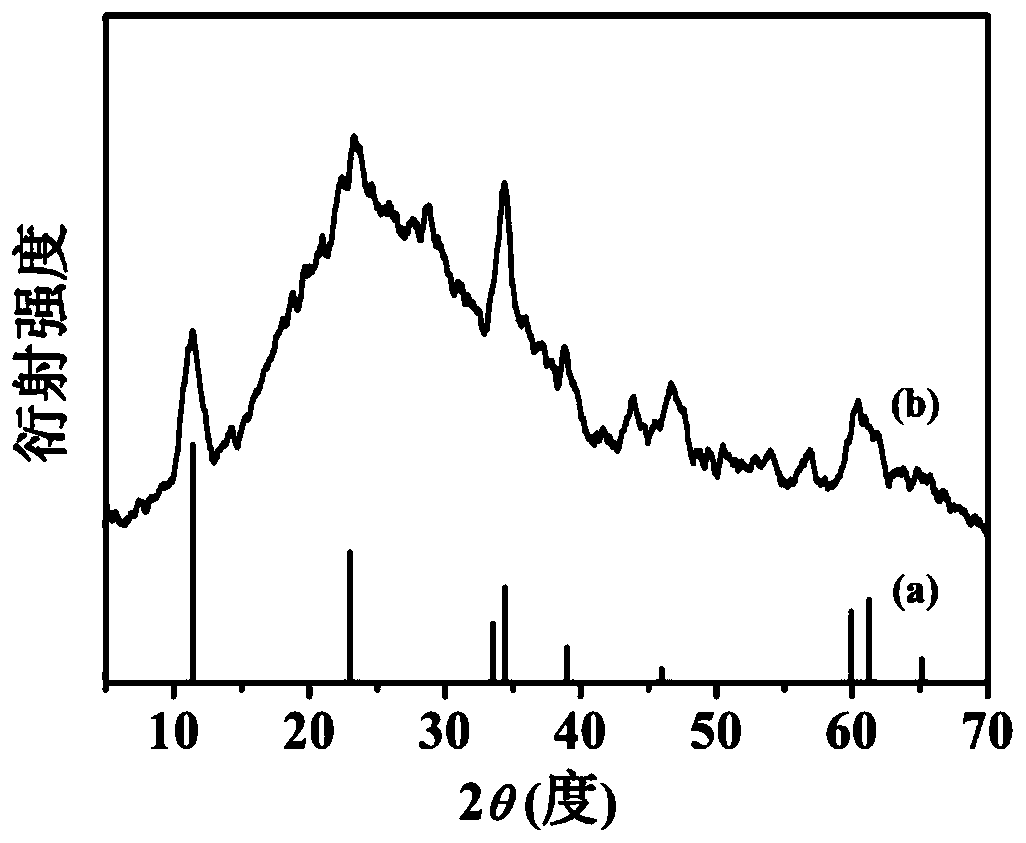
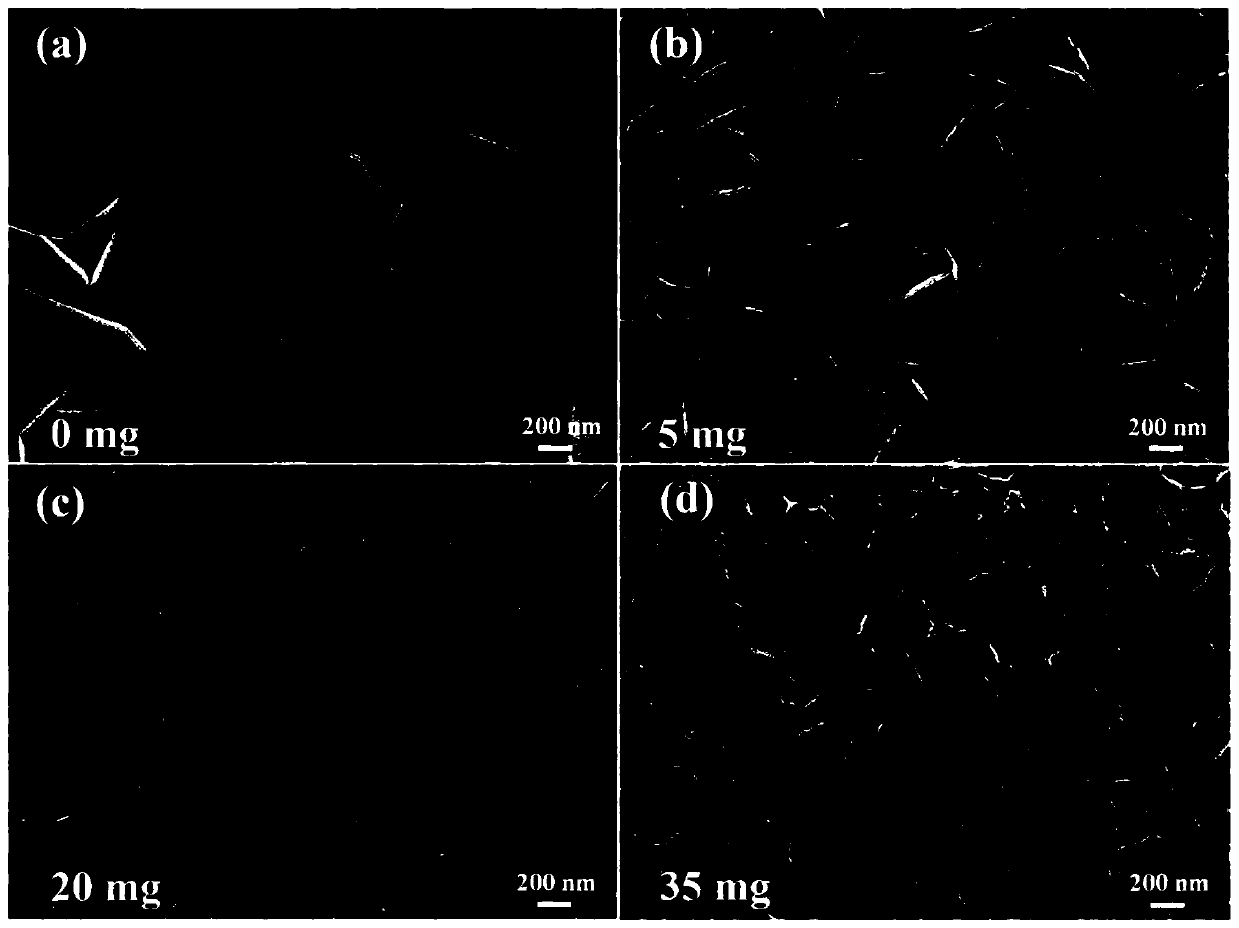
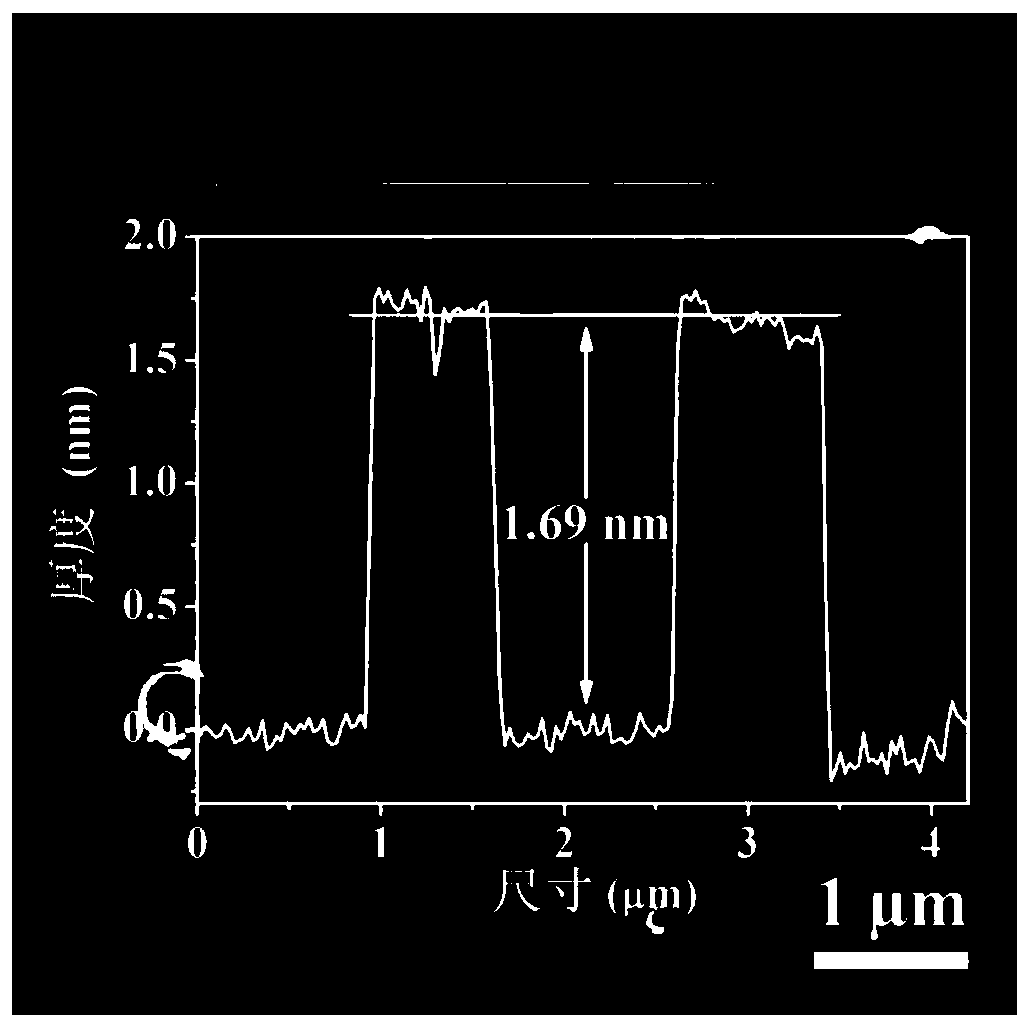
[0047] (2) Add 20.0mg 2,5-dihydroxyterephthalic acid, 15mL hydrochloric acid solution (2.0mol / L) and 15mL N,N-dimethylacetamide (DMA ) and stir evenly to form a mixed solution. Then put the stainless steel sheet pretreated in step (1) into the reactor as the growth substrate and metal source. The sealed reaction kettle was heated in an oven and kept at 150°C for 18 hours. After natural cooling to room temperature, the obtained dark green FeNiCr-LDH-SS was washed several times with ethanol and distilled water and dried at 60 °C.
Embodiment 2
[0049] (1) The pretreatment of the substrate is the same as the step (1) of Example 1.
[0050] (2) Add 15 mL of hydrochloric acid solution (2.0 mol / L) and 15 mL of N,N-dimethylacetamide (DMA) into a polytetrafluoroethylene reactor (50 mL) and stir evenly to form a mixed solution. Then the pretreated stainless steel sheet was put into the reactor as the growth substrate and metal source. The sealed reaction kettle was heated in an oven and kept at 150°C for 18 hours. After natural cooling to room temperature, the obtained dark green FeNiCr-LDH-SS was washed several times with ethanol and distilled water and dried at 60 °C.
Embodiment 3
[0052] (1) The pretreatment of the substrate is the same as the step (1) of Example 1.
[0053] (2) Add 5mg of 2,5-dihydroxyterephthalic acid, 15mL of hydrochloric acid solution (2.0mol / L) and 15mL of N,N-dimethylacetamide (DMA) into a polytetrafluoroethylene reactor (50mL) And stir evenly to form a mixed solution. Then the pretreated stainless steel sheet was put into the reactor as the growth substrate and metal source. The sealed reaction kettle was heated in an oven and kept at 150°C for 18 hours. After natural cooling to room temperature, the obtained dark green FeNiCr-LDH-SS was washed several times with ethanol and distilled water and dried at 60 °C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com