Polyketone compound pyoluterin, and preparation method and application thereof
A compound, the technology of the target compound, applied in the preparation of anticancer drugs, polyketide pyoluteorin and its preparation field, can solve the problem of less research on Aspergillus niger compounds, achieve significant cytotoxic activity, rich resources, and diverse sources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of polyketide pyoluteorin
[0036] 1. Sample source
[0037] Wetland soil from Gongqing National Forest Park, Shanghai.
[0038] 2. Strain fermentation, extraction, separation and purification methods
[0039] Dilute and mix the retrieved fresh wetland soil with distilled water at a ratio of 1:100. Take 100 μL of the soil dilution and spread it on the separation medium (separation medium: improved Martin solid medium (5.0 g / L peptone, 1.0 g / L L dipotassium hydrogen phosphate, 0.5g / L magnesium sulfate, 2.0g / L yeast extract powder, 20.0g / L glucose, 14.0g / L agar, etc.), add potassium dichromate (50mg / L) and nalidixic acid (20mg / L) as a bacteriostatic agent.), at a temperature of 28 ° C, cultivated for 4 days, after the bacterial strain grew out, it was picked and purified to obtain Aspergillus niger (Aspergillus niger) bacterial strain .
[0040] The above-mentioned purified Aspergillus niger strain was inoculated on the improved Martin s...
Embodiment 2
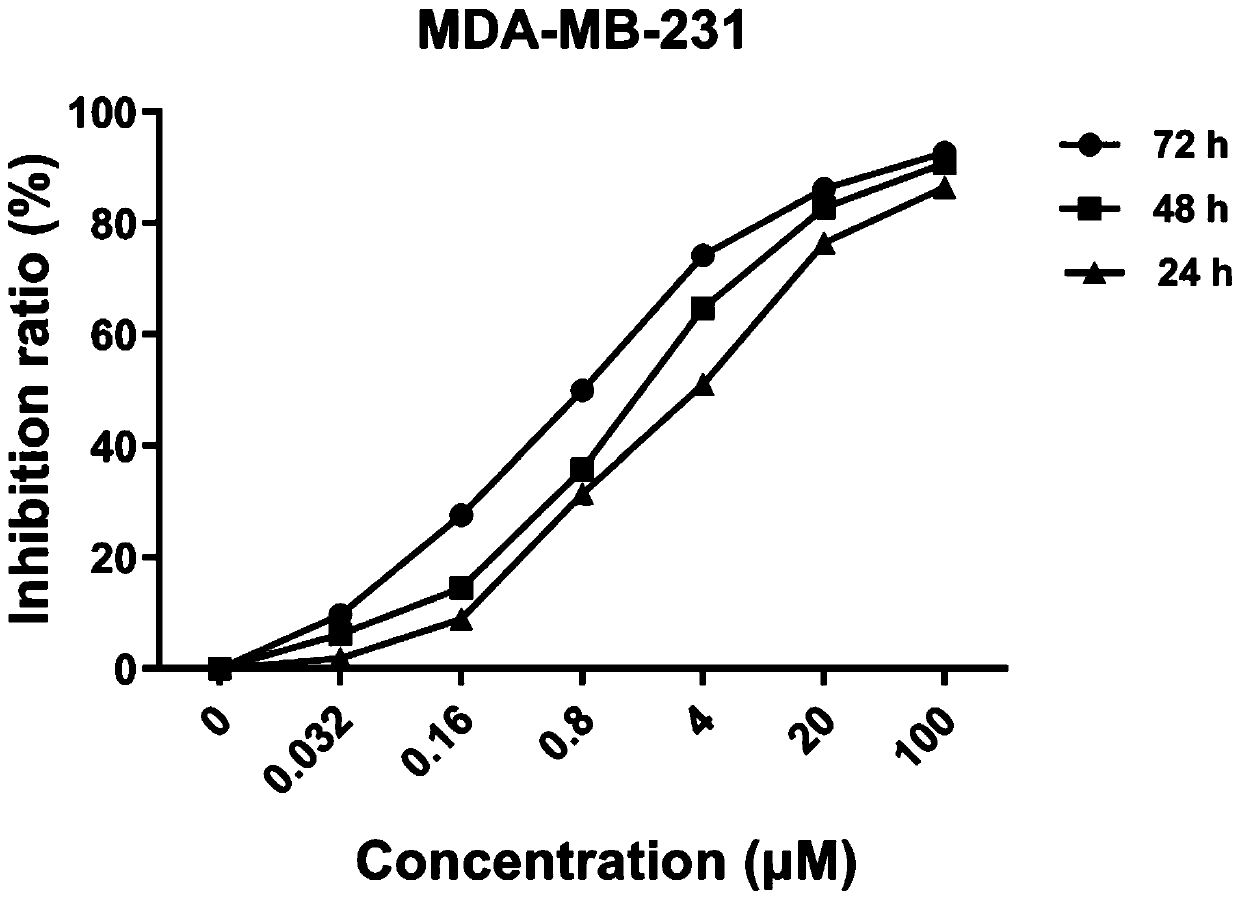
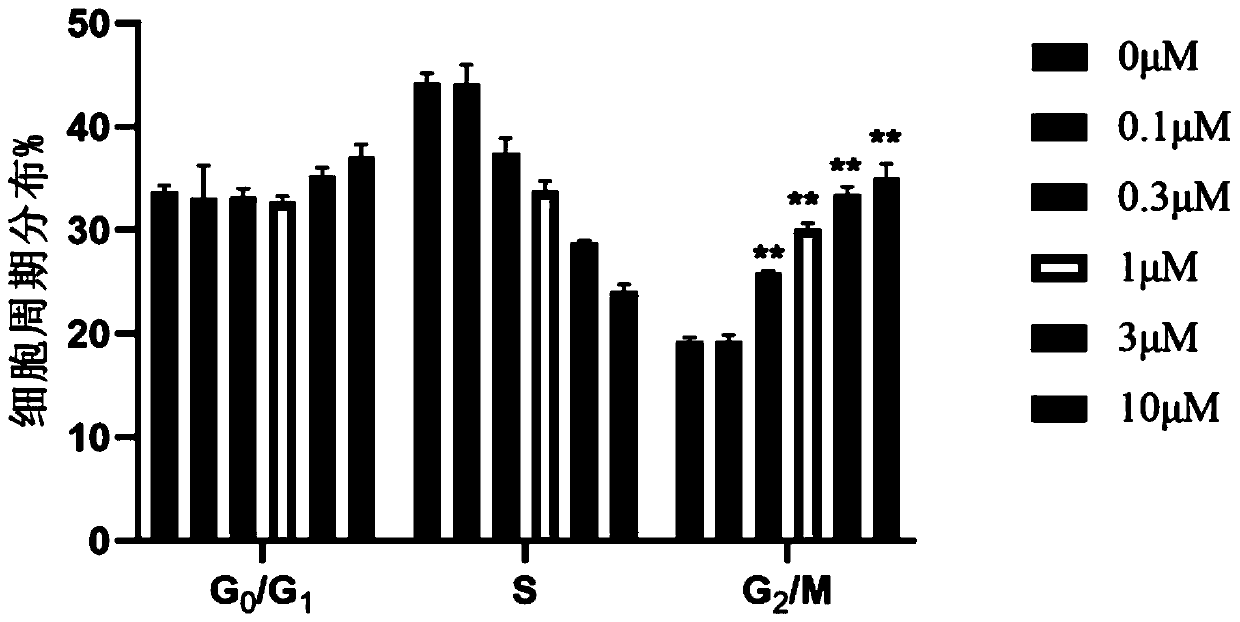
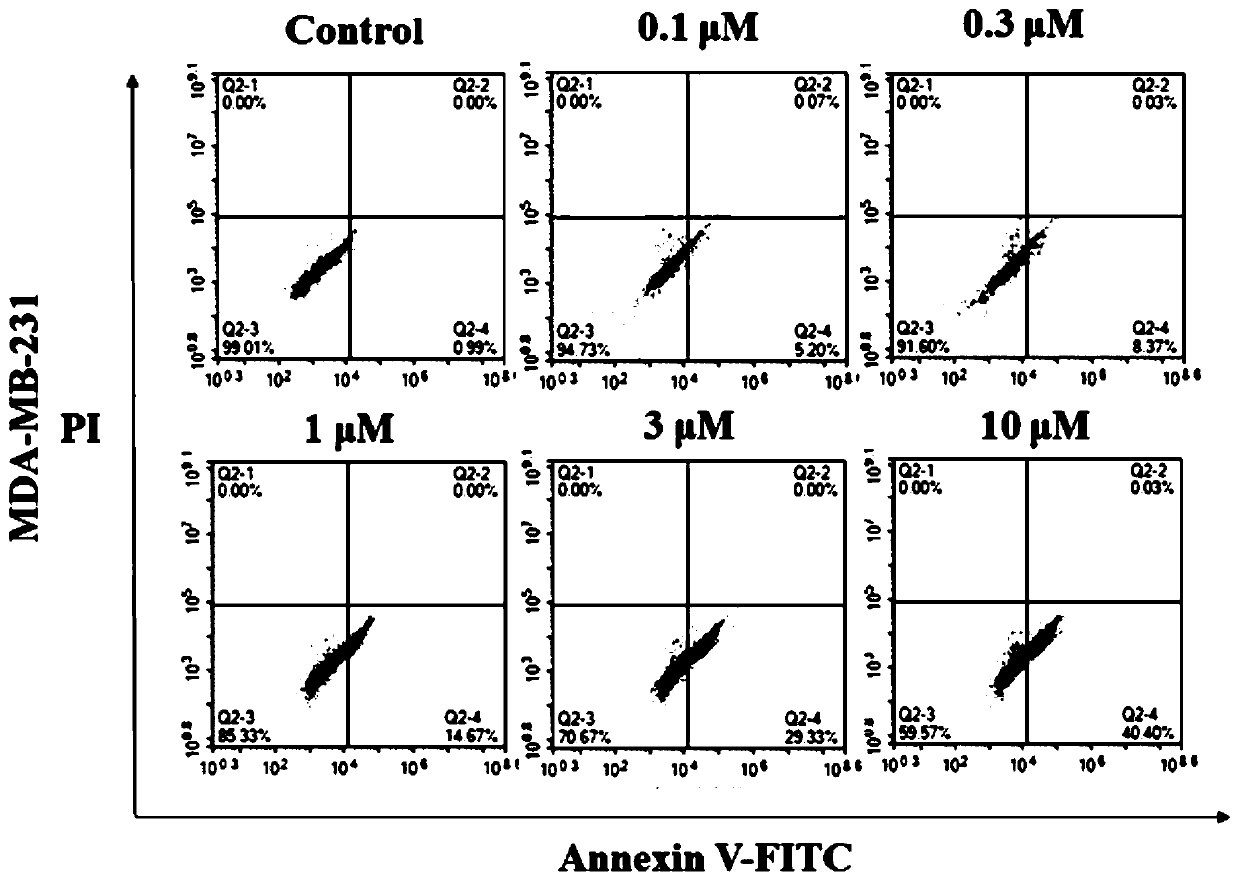
[0046] Example 2: Cytotoxic activity test
[0047] 1. Experimental materials
[0048] Human glioma cells (U87), human lung adenocarcinoma cells (A549), human colon cancer cells (HCT116), human breast cancer cells (MCF-7, HCC1954), human chronic myelogenous leukemia cells (K562), human Triple-negative breast cancer cells (MDA-MB-231, MDA-MB-468), human triple-positive breast cancer cells (BT474) and human normal breast epithelial cells (MCF-10A) were purchased from ATCC.
[0049] The polyketide compound pyoluteorin was prepared from Example 1.
[0050] 2. Cytotoxic activity screening and mechanism research methods
[0051] (1) CTG method to detect cytotoxic activity
[0052] Different tumor cells growing in the logarithmic phase were collected, counted and inoculated in 96-well culture plate, 2000 cells / well, and 90 μL DMEM culture solution (4.5 g / L D-glucose, L-glutamic acid, 110 mg / well) was added to each well. L sodium pyruvate, etc., containing serum), 37 ° C, 5% CO 2 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap