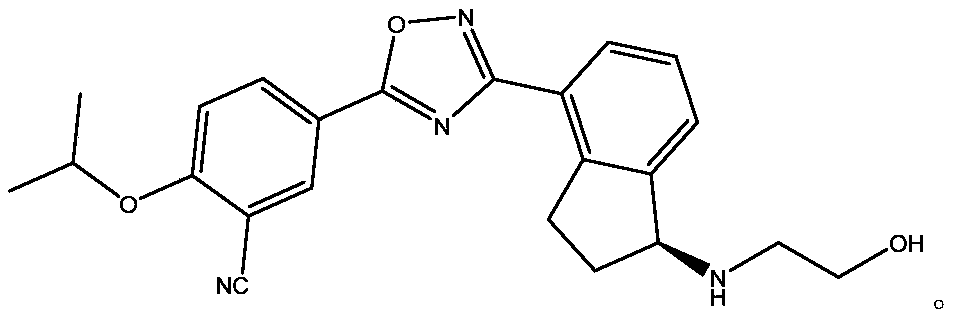
Preparation method of indanone intermediate
A technology for intermediates and indanones, applied in the field of preparation of indanone intermediates, can solve the problems of deadly toxicity, high material cost, and restricting large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
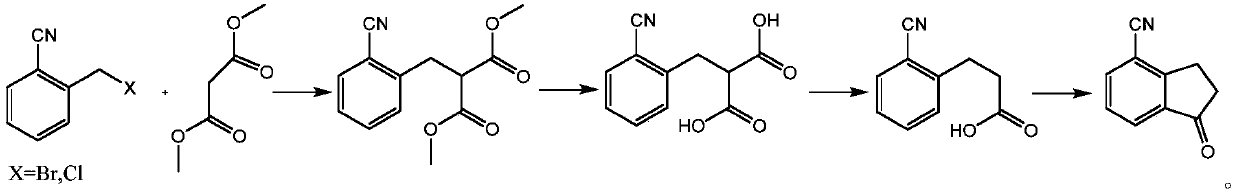
[0017] According to one aspect of the present invention, the present invention proposes a kind of preparation method of intermediate 2-(2-cyanobenzyl) dimethyl malonate, comprising the following steps:
[0018] (1) In an organic solvent, under the action of a base and a catalyst, 2-cyanobenzyl bromide or 2-cyanobenzyl chloride reacts with dimethyl malonate at a certain temperature, the reaction is completed, and after post-treatment, to obtain Dimethyl 2-(2-cyanobenzyl)malonate.
[0019] According to one aspect of the present invention, the present invention proposes a kind of preparation method of intermediate 2-(2-cyanobenzyl) malonic acid, comprising the following steps:
[0020] (2) Dimethyl 2-(2-cyanobenzyl)malonate is reacted in an organic solvent under the action of an aqueous alkali solution, and after post-treatment, 2-(2-cyanobenzyl)malonate is obtained.
[0021] In some embodiments, according to one aspect of the present invention, a kind of preparation method of i...
specific Embodiment approach
[0103] Embodiments of the present invention are described in detail below. The embodiments described below are exemplary only for explaining the present invention and should not be construed as limiting the present invention. If no specific technique or condition is indicated in the examples, it shall be carried out according to the technique or condition described in the literature in this field or according to the product specification. The reagents or instruments used were not indicated by the manufacturer, and they were all commercially available conventional products.
[0104] In order to describe the present invention, examples are listed below. However, it should be understood that the present invention is not limited to these examples, but only provides a method of practicing the present invention.
[0105] In the examples described below, unless indicated otherwise, all temperatures are in degrees Celsius. Unless otherwise stated, all reagents and solvents used wer...
Embodiment 1
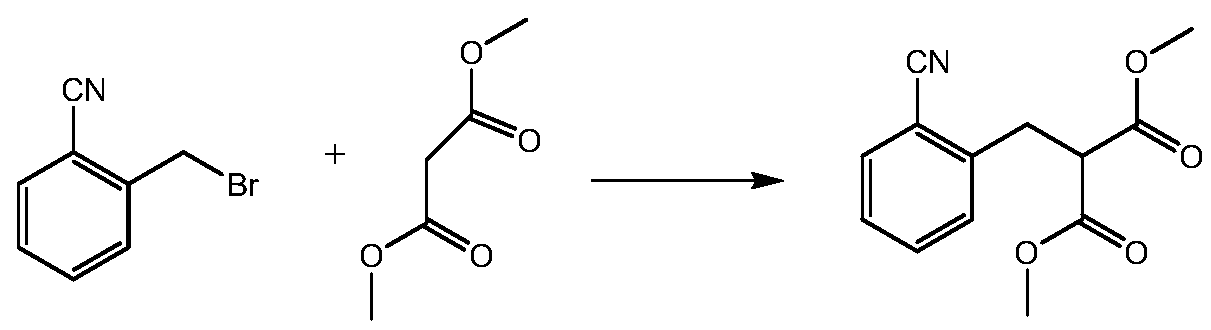
[0106] Embodiment 1: Preparation 2-(2-cyanobenzyl) dimethyl malonate
[0107] The reaction formula is as follows:
[0108]
[0109] Add 2-cyanobenzyl bromide (20.0g), acetonitrile (200mL), salt of wormwood (28.20g), sodium iodide (3.06g), dimethyl malonate (26.96g) successively in single-necked bottle; Stir at 60°C; after the reaction, cool the system to 30°C, distill under reduced pressure, add 200mL water and 200mL dichloromethane, collect the dichloromethane layer, wash once with water, collect the dichloromethane layer and concentrate to obtain 2-(2-cyano Benzyl) dimethyl malonate 32.62g, purity 80%, yield 100%.
[0110] LC-MS:m / z(ESI):248.20(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com