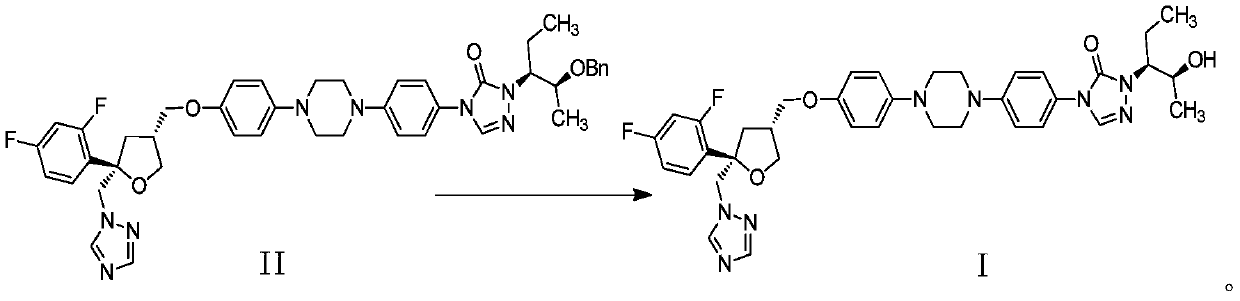
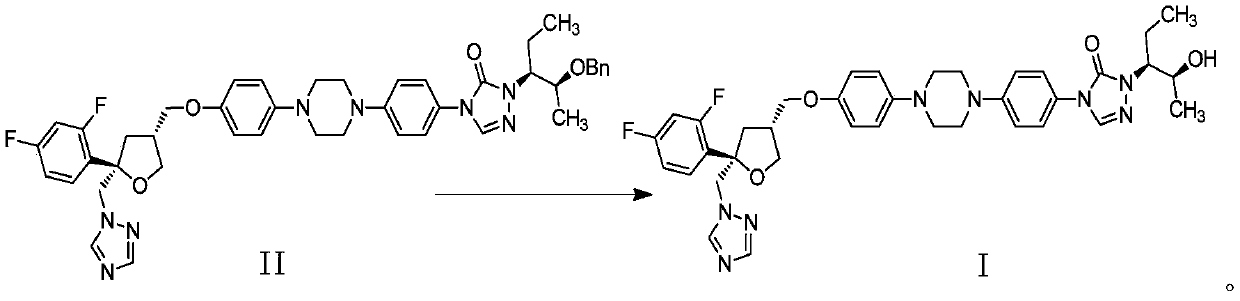
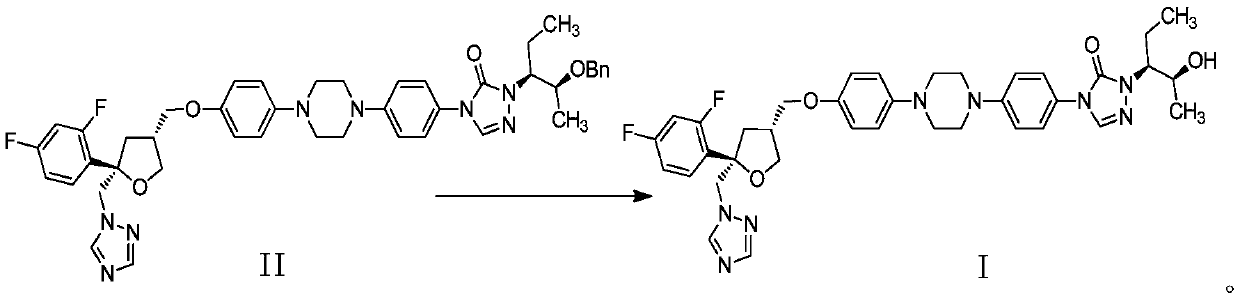
Preparation method of Noxafil
The technology of a posaconazole and a purification method is applied in the field of preparation of posaconazole, can solve the problems of many by-products, low total yield, complicated operation, etc., and achieves few reaction by-products, no heavy metal residues, and simple operation. safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of phenyl (4-(4-(4-hydroxyl)-1-piperazinyl) phenyl) carbamate (V)
[0030]
[0031] Add 4.00kg of 1-(4-aminophenyl)-4-(4-hydroxyphenyl)piperazine (VII) and 35.57kg of tetrahydrofuran into a 100L glass reactor, start stirring, and cool down to 0-10°C. Keep the temperature below 25°C and add 2.56 kg of phenyl chloroformate (VIII) dropwise, and the dropwise addition is completed in 0.5-1 hour, and the reaction is continued for 0.5-1.0 hour under temperature control at 20-25°C. The reaction was monitored by TLC. After the reaction was completed, the temperature was lowered to 0-10°C, and saturated aqueous sodium bicarbonate solution (2.00 kg of sodium bicarbonate dissolved in 38 kg of purified water) was added, followed by 20 kg of purified water, and stirred for 10-20 min. Centrifugal filter until almost no solvent flows out, then rinse with 8kg of purified water, and centrifugal filter until basically no solvent flows out.
[0032]The whole b...
Embodiment 2
[0033] Example 2: 2-((2S,3S)-2-(benzyl)-3-pentyl)-4-(4-(4-(4-hydroxyl)-1-piperazine)phenyl)-2 , Preparation of 4-dihydro-3-hydro-1,2,4-triazol-3-one (III)
[0034]
[0035] Add 4.36kg N'-((2S,3S)-2-benzyloxy)pentyl-3-formylhydrazide oxalate (VI) and 45.36kg dioxane into a 100L glass reactor, start stirring, Add 3.45 kg N,N-diisopropylethylamine. After stirring for 1-1.5 hours, 5.47 kg of phenyl (4-(4-(4-hydroxy)-1-piperazinyl)phenyl)carbamate (V) was added. After the addition, the temperature was raised to 80±5°C for 24-30 hours. TLC monitoring. After the reaction was completed, the temperature of the system was lowered to 15-25° C., 57.79 kg of dichloromethane and 21.81 kg of pure water were added, stirred for 10-20 minutes, and the layers were separated. The lower organic phase was collected, and the aqueous phase was discarded. The organic phase was transferred to a 100L glass reactor, and 21.81 kg of purified water was added to the reactor, stirred for 10-20 minutes...
Embodiment 3
[0036] Example 3: 4-(4-(4-(4-(((3R,5R)-5-((1-hydrogen-1,2,4-l-1-triazolyl)methyl)-5 -(2,4-Difluorophenyl)-3-tetrahydrofuryl)methoxy)phenyl)-1-piperazinyl)phenyl)-2-((2S,3S)-2-(benzyl) Preparation of -3-pentyl)-2,4-dihydro-3-hydro-1,2,4-triazol-3-one (II)
[0037]
[0038] With 35.01kg dimethyl sulfoxide, mass concentration is 50% sodium hydroxide aqueous solution (sodium hydroxide 1.24kg is dissolved in pure water 1.24kg, and described mass concentration refers to that the quality of sodium hydroxide accounts for the total mass of sodium hydroxide aqueous solution %), added to a 50L glass reactor, stirred to dissolve, then added 5.31kg of compound (III), stirred for 20 to 30 minutes, added 4.60Kg (3S,5R)-toluene-4-sulfonic acid 5-(2, 4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester (IV). After the feeding is completed, the temperature is controlled at 25±5°C for 8-12 hours. TLC monitors the reaction, after the reaction is complete. Contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com