Salt of bromine domain structural protein inhibitor and preparation method and application thereof
A technology of acid addition salts and alkyl groups, used in crystal forms and pharmaceutical compositions, the preparation of the above salts and crystal forms, and the field of salts of bromodomain structural protein inhibitors, which can solve the problem that tumor cells cannot be completely removed or completely killed. , tumor metastasis or recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245] The preparation of embodiment one formula (III) compound nitrate
[0246]
[0247] The first step: 4-bromo-7-methoxy-2-methyl-1H-pyrrolo[2,3-c]pyridine
[0248]
[0249]5-Bromo-2-methoxy-3-nitropyridine (15g, 64.3mmol) was dissolved in tetrahydrofuran (150mL), and isoallylmagnesium bromide (385mL, 192.9 mmol, 0.5 M). The reaction solution was stirred at -78°C for 3 hours, the reaction solution was quenched with saturated aqueous ammonium chloride (100 mL), the solution was extracted with ethyl acetate (100 mL×2), the organic phase was washed with saturated brine (100 mL), and anhydrous sulfuric acid Sodium dry. The organic phase was dried and spin-dried, and column separation (petroleum ether / ethyl acetate=5 / 1) gave 4-bromo-7-methoxyl-2-methyl-1H-pyrrolo[2,3-c]pyridine (5.5 g, yellow oil).
[0250] 1 H NMR (400MHz, CDCl 3 ) δ 7.80 (d, J = 5.6Hz, 1H), 7.26 (s, 1H), 4.06 (s, 3H), 2.48 (s, 3H).
[0251] The second step: 4-bromo-7-methoxy-2-methyl-1-toluenesulf...
Embodiment 2
[0290] The preparation of embodiment two formula (III) compound crystal form
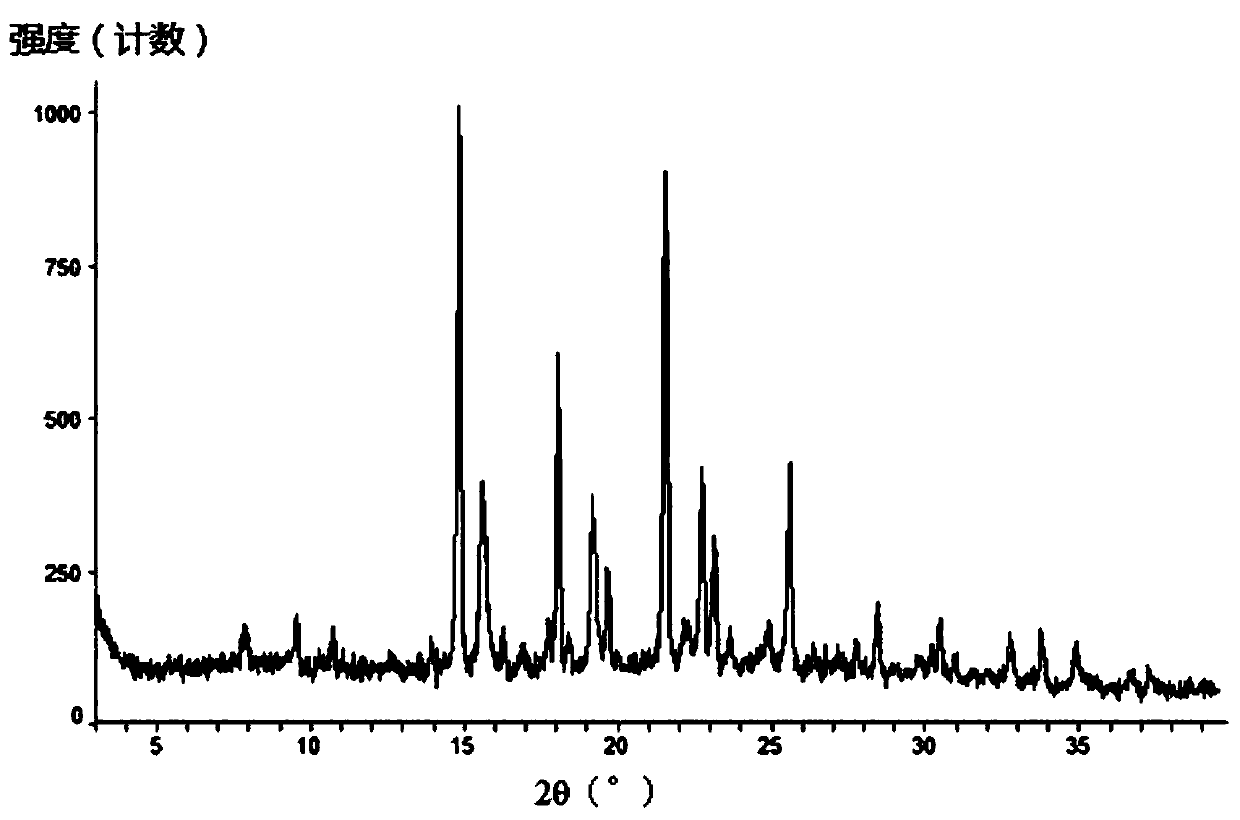
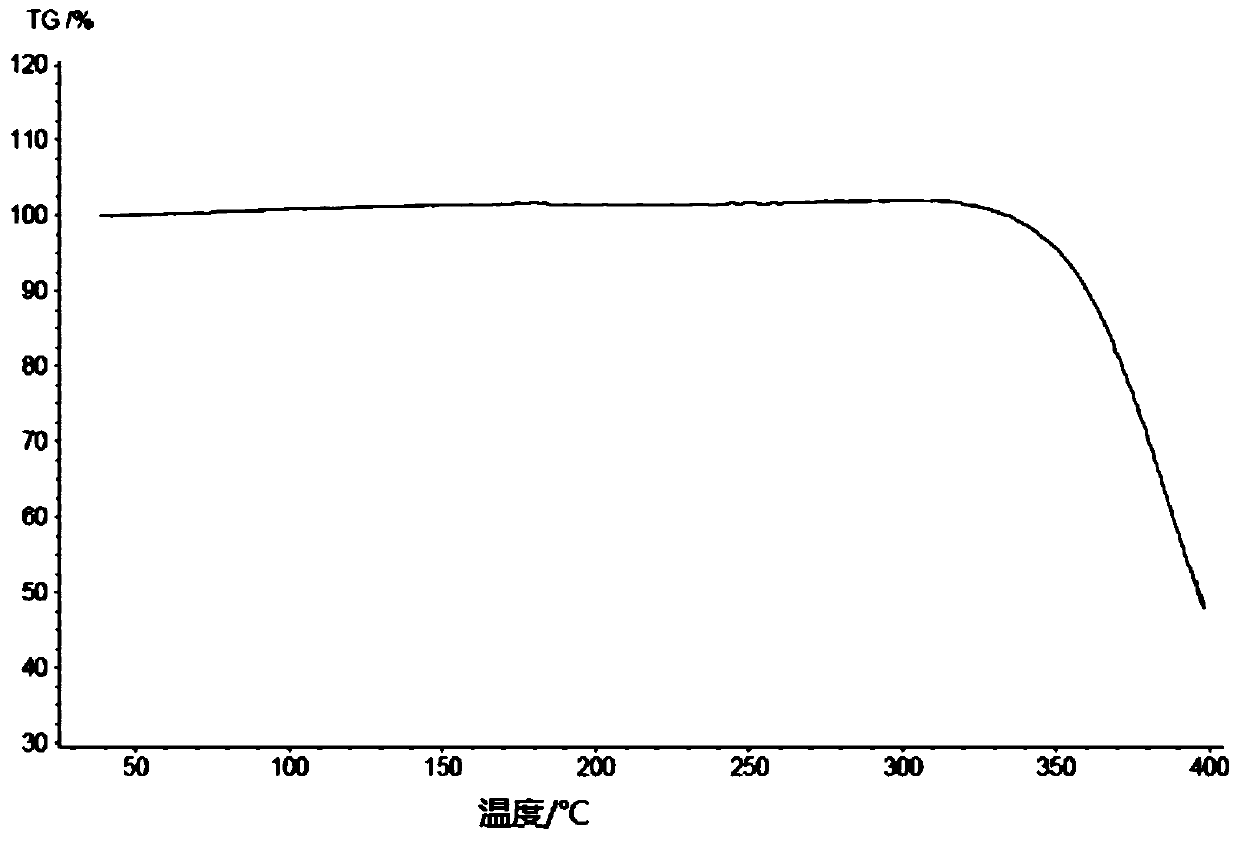
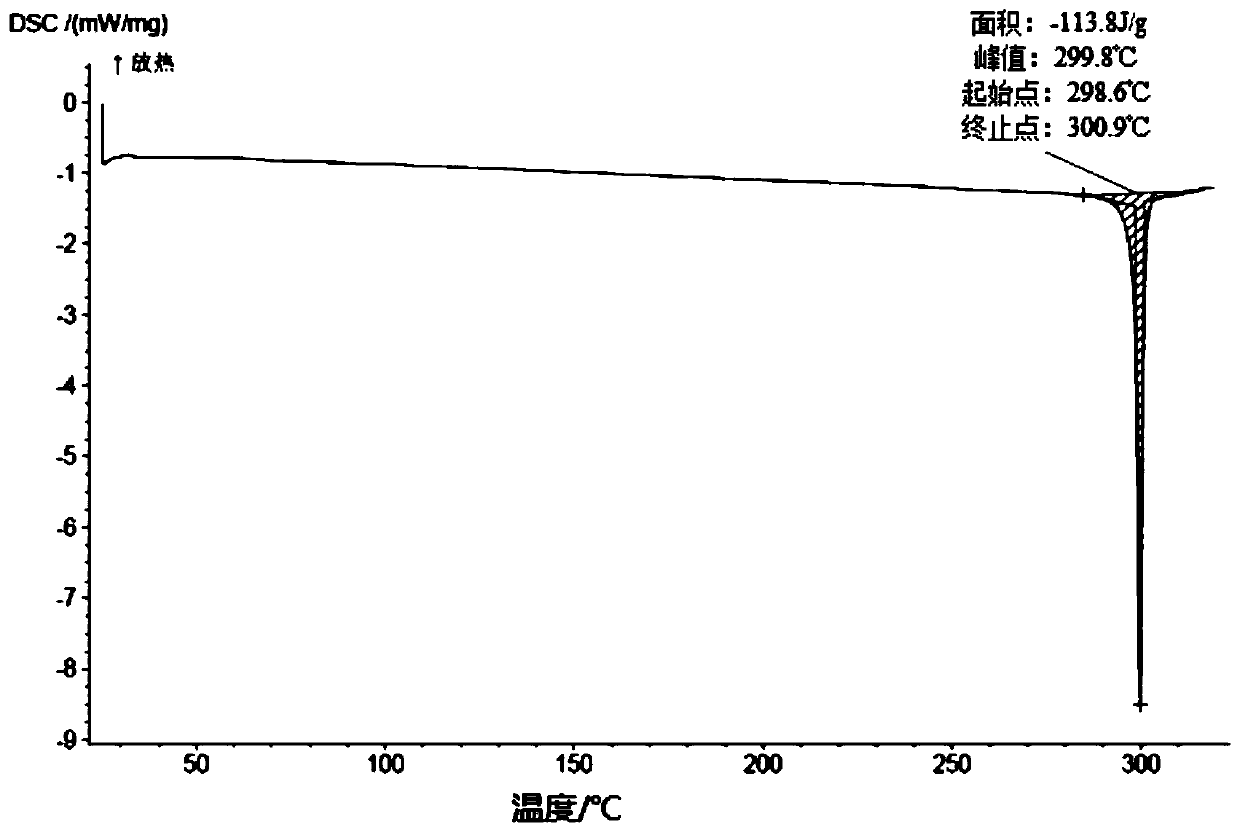
[0291] Add 10.7 g of the compound of formula (III) into 18 ml of dichloromethane, and stir until it dissolves. Add 64ml of acetonitrile to the solution, stir at room temperature to precipitate a solid, stir at room temperature for 3h, filter, and rinse with 24ml of acetonitrile. Finally, the solid was dried at 35°C until constant weight, and finally an off-white solid was obtained. After testing and analysis, it has basic figure 1 The XRPD pattern shown, such as figure 2 shown in the TGA plot as well as image 3 DSC plot shown.
Embodiment 3
[0292] The preparation of embodiment trinitrate crystal form I
[0293] Weigh 40mg of the compound of formula (III), add 2mL of methanol to dissolve it, and filter it through a membrane filter. Add 96uL of 1M ethanol solution of nitric acid to the filtrate, stir at room temperature for 10min without solid formation, put the solution at 50°C and stir to volatilize. Add 2.5mL of Ethyl acetate solution until the solid was precipitated, and the solid was dried in a vacuum oven at 40°C to constant weight to obtain the nitrate salt crystal form I. After testing and analysis, it has basically Figure 4 The XRPD pattern shown, such as Figure 5 shown in the TGA graph and as Image 6 DSC plot shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com