Stannous chloride dehydrate and preparation method thereof
A stannous chloride, dihydrate technology, applied in the directions of stannous chloride, tin halide, etc., can solve the problems of long reaction time, short process route, high reaction temperature, reduce the temperature required for the reaction, shorten the reaction time, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
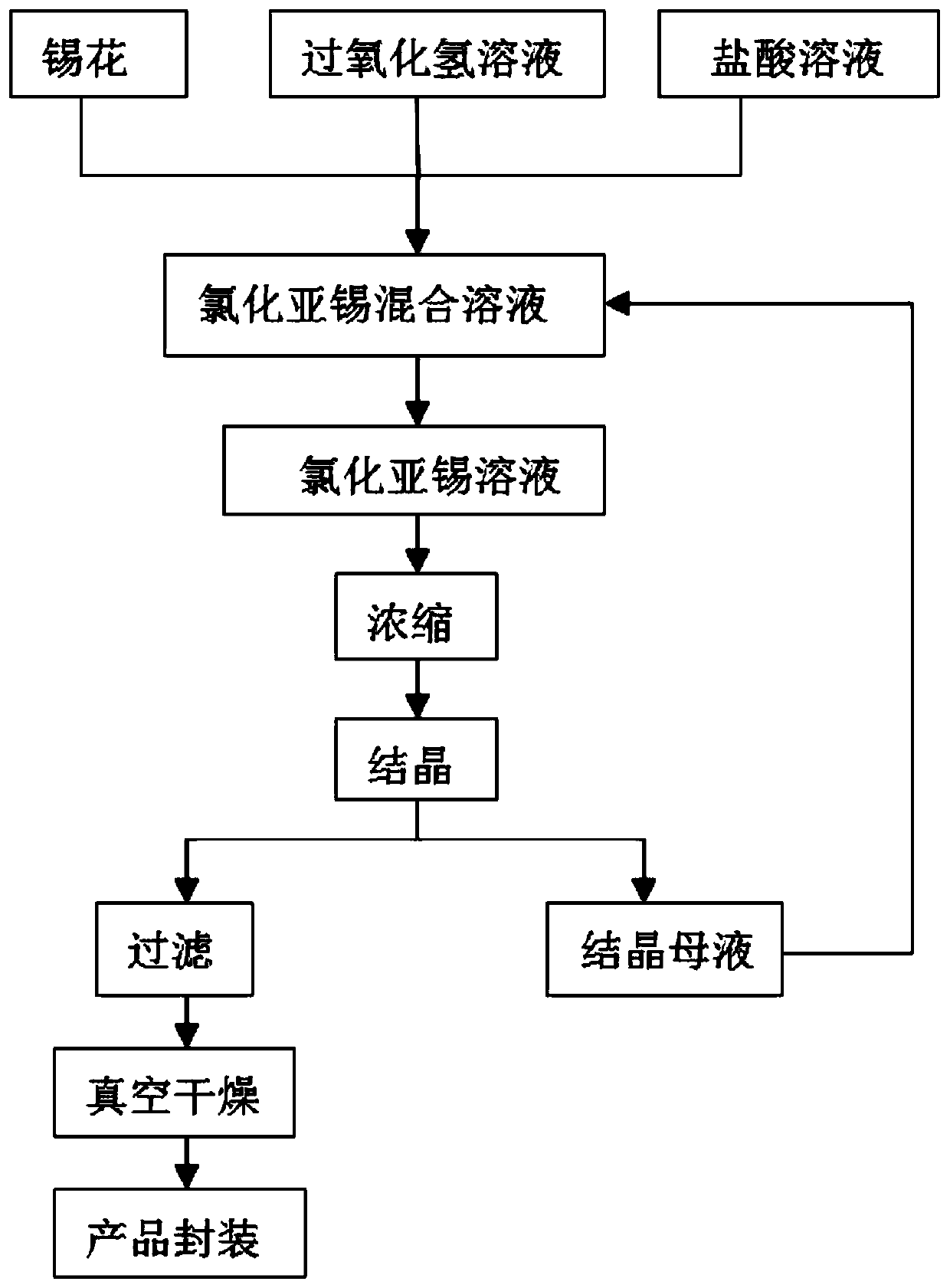
[0024] The invention provides a kind of preparation method of tin protochloride dihydrate, it comprises the steps:
[0025] (1) melting and quenching a tin ingot with a purity of 3.5N into tin flowers with a size of 1 to 10 mm;
[0026] (2) Add a certain amount of tin flower in the reaction vessel, then add an appropriate amount of hydrochloric acid solution with a mass fraction of 10% to 20% to submerge the tin flower, control the reaction temperature at 40 to 70°C, and then drop in a mass fraction of 10% to 20%. 30% hydrogen peroxide solution, react 3~8h, make tin flower dissolve, obtain the mixed solution that contains tin dichloride and tin tetrachloride; Wherein, the consumption ratio of hydrogen peroxide solution and tin flower is V(H 2 o 2 ): W(Sn)=2:1~4:1(mL / g), the rate of addition of hydrogen peroxide solution is 200~500mL / h;
[0027] (3) Add excessive tin flower in the mixed solution of step (2), react 4~8h, reduce the Sn in the solution 4+ , filter after the rea...
Embodiment 1
[0034] 500g tin flower and 1.5L hydrochloric acid solution (mass fraction 10%) were placed in the reactor, and 1000mL H was added dropwise at a speed of 200mL / h 2 o 2 solution (mass fraction 30%), after reacting at 70° C. for 5 hours, the tin flowers were completely dissolved to obtain a mixed solution containing tin dichloride and tin tetrachloride. Add excess tin flower to the above mixed solution to reduce Sn 4+ , after reacting for 5 hours, filter, adjust the pH of the filtrate to 1.5, and then concentrate under reduced pressure. When the density of the concentrated solution reaches 2.3g / mL (2.0-2.3g / mL is acceptable), the concentration is stopped, the concentrated solution is placed at 0°C (0-5°C is acceptable) for crystallization for 12 hours, and the crystal is placed under nitrogen protection Suction filtration under the atmosphere to remove the liquid in the upper layer of the crystals, and place the separated crystals in a vacuum oven at -0.9 bar (-0.75 to -0.9 bar...
Embodiment 2
[0037] 500g tin flower and 1.5L hydrochloric acid solution (mass fraction 15%) were placed in the reactor, and 1500mL H was added dropwise at a speed of 200mL / h 2 o 2 solution (mass fraction 20%), after reacting at 55° C. for 7.5 hours, the tin flower completely dissolves to obtain a mixed solution containing tin dichloride and tin tetrachloride. Add excess tin flower to the above mixed solution to reduce Sn 4+ , after reacting for 5 hours, filter, adjust the pH of the filtrate to 1.0, and then concentrate under reduced pressure. When the density of the concentrated solution reaches 2.3g / mL (2.0-2.3g / mL is acceptable), the concentration is stopped, the concentrated solution is placed at 0°C (0-5°C is acceptable) for crystallization for 12 hours, and the crystal is placed under nitrogen protection Suction filtration under the atmosphere to remove the liquid in the upper layer of the crystals, and place the separated crystals in a vacuum oven at -0.9 bar (-0.75 to -0.9 bar is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com