A kind of preparation method of high-purity thalidomide alpha crystal form
A technology of thalidomide and its crystal form, which is applied in the field of preparation of high-purity thalidomide α crystal form, and can solve the problems of difficult reuse of mother liquor, high social and economic costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
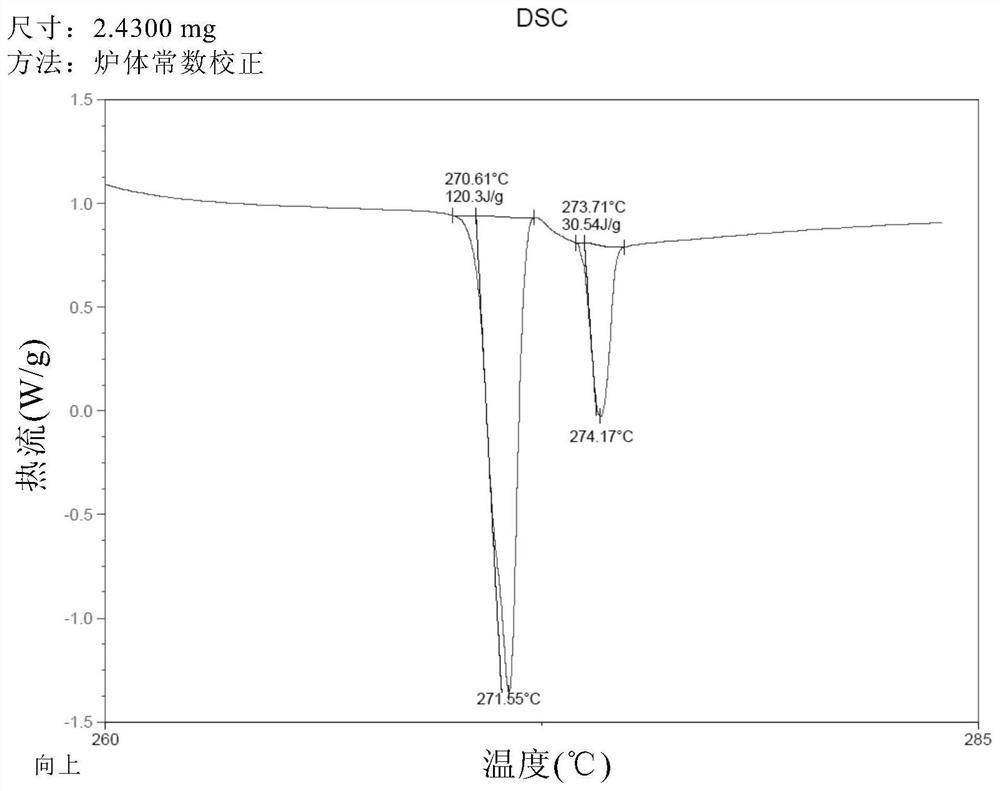
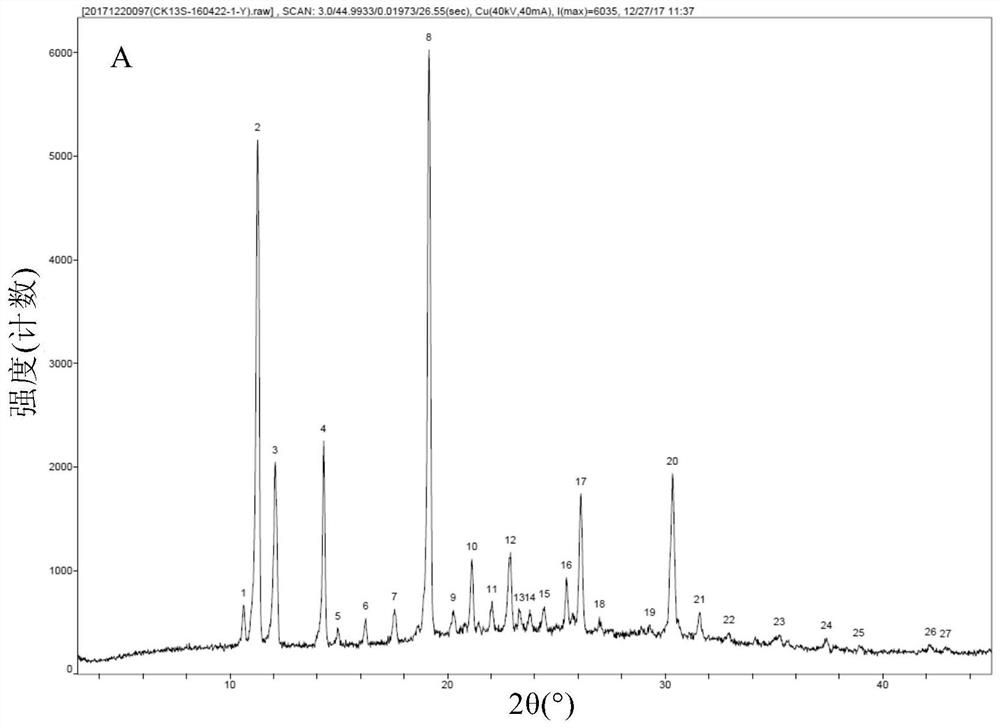
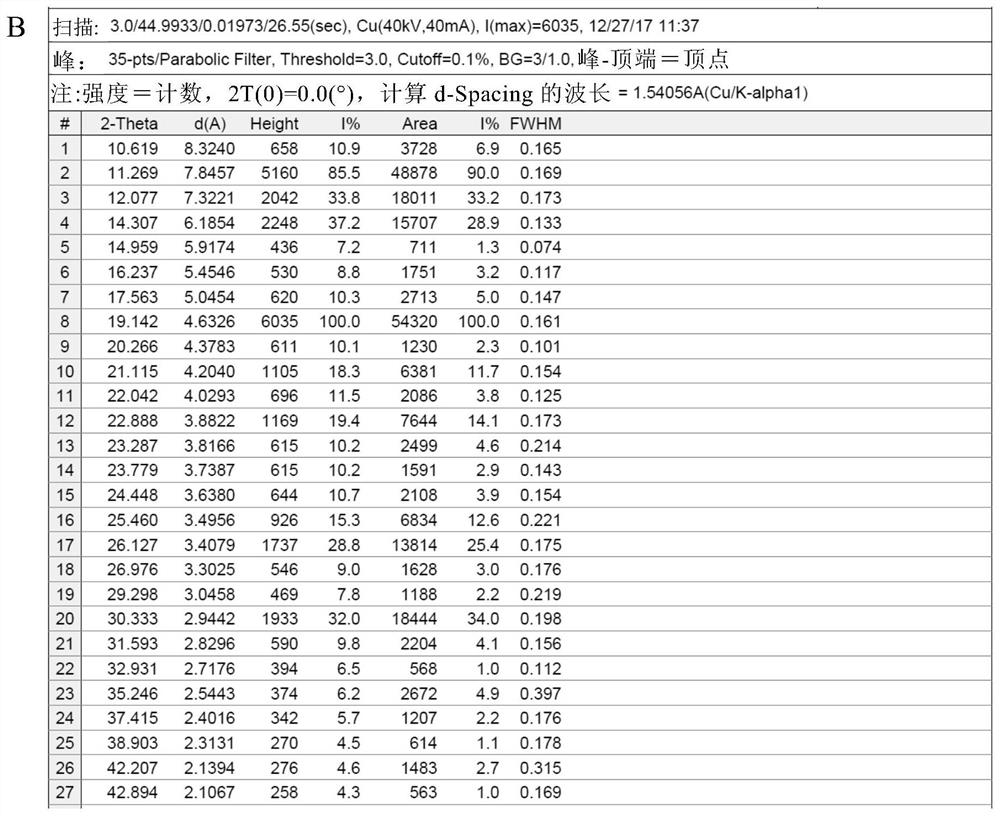
[0085] Add 50 g of crude thalidomide and 1,500 g of tetrahydrofuran into a 2L three-neck round bottom flask, stir mechanically, heat the oil bath to reflux, add 150 g of purified water, and dissolve all the solids. Keep the oil bath temperature at 95-100 ° C, and distill at atmospheric pressure Tetrahydrofuran, after recovery of tetrahydrofuran, the mother liquor was cooled to room temperature, vacuum-filtered under reduced pressure, and the filter cake was vacuum-dried at 80°C for 4 hours to obtain 48.5 g of thalidomide crystals, with a recrystallization yield of 97.0% and a purity of 99.8%. The residual amount was almost 0%. DSC test results such as figure 1 As shown, the endothermic peaks detected by DSC are 271.55° C. (the characteristic endothermic peak of the α crystal form) and 274.17° C., indicating that the obtained thalidomide crystals are of the α crystal form and have high purity. X-ray diffraction pattern (XRPD) as Figure 2A As shown, the X-ray diffraction data...
Embodiment 2
[0087] Add 50 g of crude thalidomide and 150 g of tetrahydrofuran into a 500 ml three-necked round-bottomed flask successively, stir mechanically, heat the oil bath to reflux, add 50 g of purified water, and dissolve all the solids, lower the temperature to room temperature and concentrate under reduced pressure to recover tetrahydrofuran, and the recovery of tetrahydrofuran is complete , the mother liquor was suction-filtered, and the filter cake was vacuum-dried at 80° C. for 4 hours to obtain 49.0 g of thalidomide crystal product, the recrystallization yield was 98.0%, the purity was 99.7%, and the residual tetrahydrofuran was almost 0%. DSC test results such as image 3 As shown, the endothermic peaks detected by DSC are 271.47°C and 274.32°C, indicating that the obtained thalidomide crystals are in the α crystal form and have high purity. X-ray diffraction pattern (XRPD) as Figure 4A As shown, the X-ray diffraction data as Figure 4B shown. It also shows that the obta...
Embodiment 3
[0089] Thalidomide crude product 50g, tetrahydrofuran recovered in Example 1 was weighed 1500g and added to a 2L three-necked round-bottomed flask successively, mechanically stirred, the oil bath was heated to reflux, 150g of purified water was added, all the solids were dissolved, and the mixture was reduced to Concentrate under reduced pressure at room temperature to recover tetrahydrofuran. After the recovery of tetrahydrofuran is complete, the mother liquor is suction-filtered, and the filter cake is vacuum-dried at 80°C for 4 hours to obtain 48.8 g of thalidomide crystals. The recrystallization yield is 97.6%, the purity is 99.8%, and tetrahydrofuran remains amount is almost 0%. DSC test results such as Figure 5 As shown, the endothermic peaks detected by DSC are 271.31°C (the characteristic endothermic peak of the α crystal form) and 274.23°C, indicating that thalidomide is in the α crystal form and has high purity. X-ray diffraction pattern (XRPD) as Figure 6A As sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com