Synthesis of novel covalent organic framework material and application thereof in metal ion recognition and dye adsorption
A covalent organic framework and a new technology, applied in the field of material chemistry, can solve the problems of toxic dyes, water system and human health hazards, and achieve the effects of high selectivity and sensitivity, simple synthesis method and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 0.0857g of 2,4,6-tri-(4-bromo-phenyl)-[1,3,5]-triazide and 0.1069g of pyrene-2,7-bis(4,4,5 ,5-Tetramethyl-[1,3,2]dioxaborolane), added to 10mL of N,N dimethylformamide, and ultrasonicated for 10 minutes, so that the monomer was evenly dispersed in N, In N-dimethylformamide, after degassing through three cycles, add 2.5mL saturated potassium carbonate aqueous solution and 0.0179g tetrakistriphenylphosphine palladium into the Shrek tube, vacuumize and fill with nitrogen, degas through three cycles, and use argon Purge, seal the tube, place in an oil bath at 110°C and stir for 24 hours, collect the precipitate by suction filtration, wash thoroughly with water, N,N-dimethylformamide, methanol, ethanol and dichloromethane, and wash with Tetrahydrofuran and methanol were used as solvents to pass Soxhlet extracts through reflux washing for 24 h and dry in vacuo to prepare covalent organic framework (COF) materials.
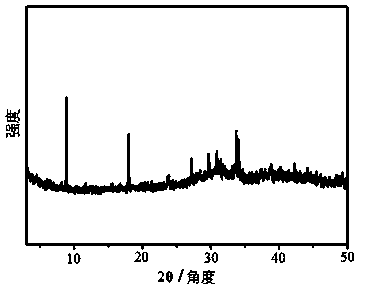
[0031] The structure morphology of the COF material t...
Embodiment 2
[0051] Weigh 0.0857g of 2,4,6-tri-(4-bromo-phenyl)-[1,3,5]-triazide and 0.1069g of pyrene-2,7-bis(4,4,5 ,5-Tetramethyl-[1,3,2]dioxaborolane), added to 20mL of 1,4-dioxane, and ultrasonicated for 10 minutes to disperse the monomer evenly in 1, In 4-dioxane, after degassing through three cycles, add 5mL saturated cesium carbonate aqueous solution and 0.0179g tetrakistriphenylphosphine palladium into the Shrek tube, vacuumize and fill with nitrogen, degas through three cycles, blow with argon Sweep, seal the tube, stir in an oil bath at 90°C for 21 hours, collect the precipitate by suction filtration, wash thoroughly with water, N,N-dimethylformamide, methanol, ethanol and dichloromethane, wash with tetrahydrofuran The covalent organic framework (COF) material was prepared by refluxing the Soxhlet extract with methanol as a solvent for 24 h and drying in vacuo.
Embodiment 3
[0053] Weigh 0.0857g of 2,4,6-tri-(4-bromo-phenyl)-[1,3,5]-triazide and 0.1069g of pyrene-2,7-bis(4,4,5 ,5-Tetramethyl-[1,3,2]dioxaborolane), added to 30mL of toluene, and ultrasonicated for 10 minutes, so that the monomer was evenly dispersed in toluene, after three cycles of degassing , add 7.5mL saturated aqueous sodium carbonate solution and 0.0179g tetrakistriphenylphosphine palladium into the Shrek tube, vacuumize and fill with nitrogen, degas through three cycles, blow with argon, seal the tube, and place it in an oil bath at a temperature of 120°C Stir in the pot for 18 hours, collect the precipitate by suction filtration, wash thoroughly with water, N,N-dimethylformamide, methanol, ethanol and dichloromethane, and use tetrahydrofuran and methanol as solvents to wash back through the Soxhlet extract for 24 hours , and dried in vacuum to prepare covalent organic framework (COF) materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com