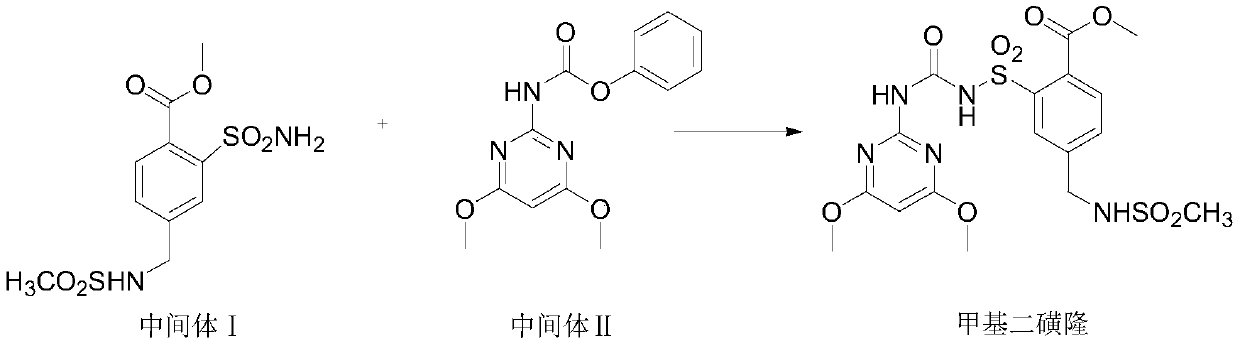
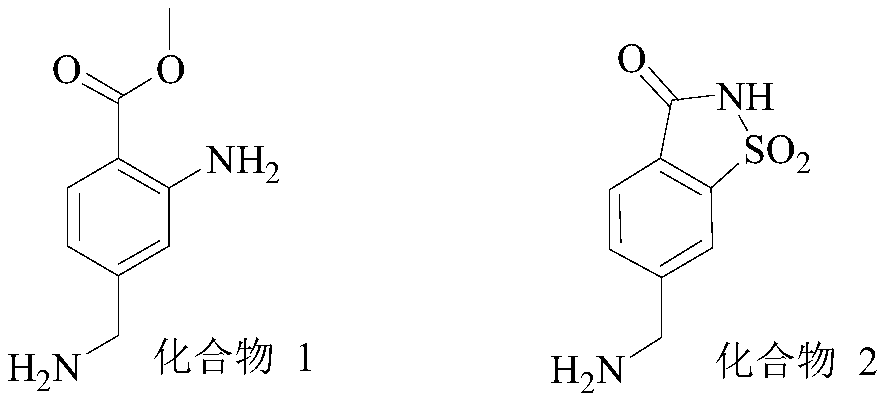
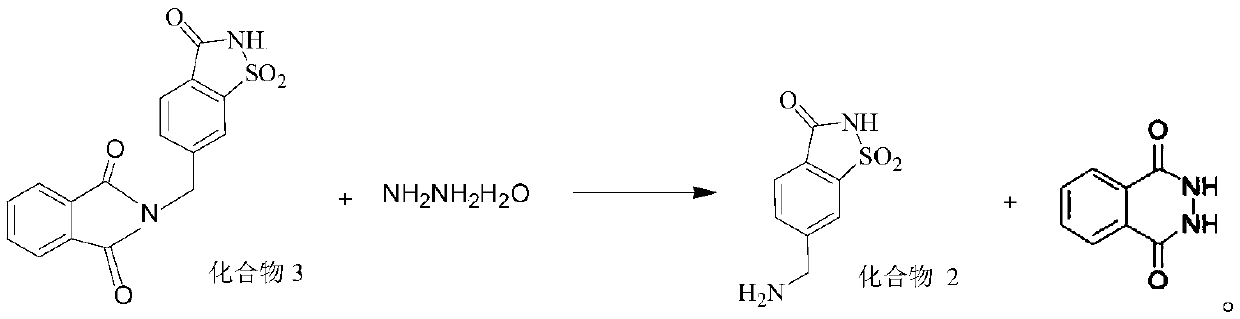
Synthesis method of 6-aminomethyl-1,1-dioxo-1,2-benzothiazol-3-one
A technology of benzothiazole and aminomethyl, which is applied in the field of organic drug synthesis, can solve problems such as troublesome synthesis methods and low yields, and achieve the effects of reducing production costs, high yields and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This example provides a synthetic method for 6-aminomethyl-1,1-dioxo-1,2-benzothiazol-3-one, including sequentially synthesizing compound 6, compound 5, compound 4, compound 3 and the target Compound steps. The specific synthesis method of each compound is shown below.
[0045] Synthesis of compound 6: 2-(3-sulfo-4-methylbenzyl)-1H-isoindole-1,3-dione
[0046] Weigh 7g of p-methylbenzyl chloride and dissolve it with an appropriate amount of N,N-dimethylformamide, and then add 7.35g of phthalimide, 8.1g of tetrabutylammonium bromide and 5.3g of sodium carbonate to the system , reacted at a temperature of 50° C. for 2 hours; after the reaction was completed, extracted with water, collected the organic layer and removed the solvent to obtain 12.3 g of 2-(4-methylbenzyl)-1H-isoindole-1,3-dione;
[0047] Dissolve 12.3g of 2-(4-methylbenzyl)-1H-isoindole-1,3-dione in chloroform, then add 8.7g of chlorosulfonic acid, and react at 80°C for 3h; after the reaction, wash with ...
Embodiment 2
[0067] This example provides a synthetic method for 6-aminomethyl-1,1-dioxo-1,2-benzothiazol-3-one, including sequentially synthesizing compound 6, compound 5, compound 4, compound 3 and the target Compound steps. The synthesis method of each compound provided in this example is basically the same as that of the corresponding compound provided in Example 1.
[0068] Synthesis of compound 6: 2-(3-sulfo-4-methylbenzyl)-1H-isoindole-1,3-dione
[0069] The method for synthesizing compound 6 in this example is basically the same as the method for synthesizing compound 6 provided in Example 1, the main difference is that the reaction solvent, basic catalyst and reaction parameters are different. Specifically, in this example, 7g of p-methylbenzyl chloride was weighed and dissolved with an appropriate amount of pyridine, and 7.35g of phthalimide, 16g of tetrabutylammonium bromide and 6g of sodium hydroxide were sequentially added to the system, React at 100°C for 1 hour; after th...
Embodiment 3
[0081] This example provides a synthesis method of 6-aminomethyl-1,1-dioxo-1,2-benzothiazol-3-one, including the steps of sequentially synthesizing compound 6, compound 4, compound 3 and the target compound . The specific synthesis method of each compound is shown below.
[0082] Synthesis of compound 6: 2-(3-sulfo-4-methylbenzyl)-1H-isoindole-1,3-dione
[0083] The method for synthesizing compound 6 in this example is basically the same as the method for synthesizing compound 6 provided in Example 1, the main difference is that the reaction solvent, basic catalyst and reaction parameters are different. Specifically, in this example, 7g of p-methylbenzyl chloride was weighed and dissolved with an appropriate amount of dimethyl sulfoxide, and 7.35g of phthalimide, 3.5g of tetrabutylammonium bromide and 6g of triethylamine was reacted at 70°C for 1.5h; after the reaction was completed, it was extracted with water, and the organic layer was collected to remove the solvent to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com