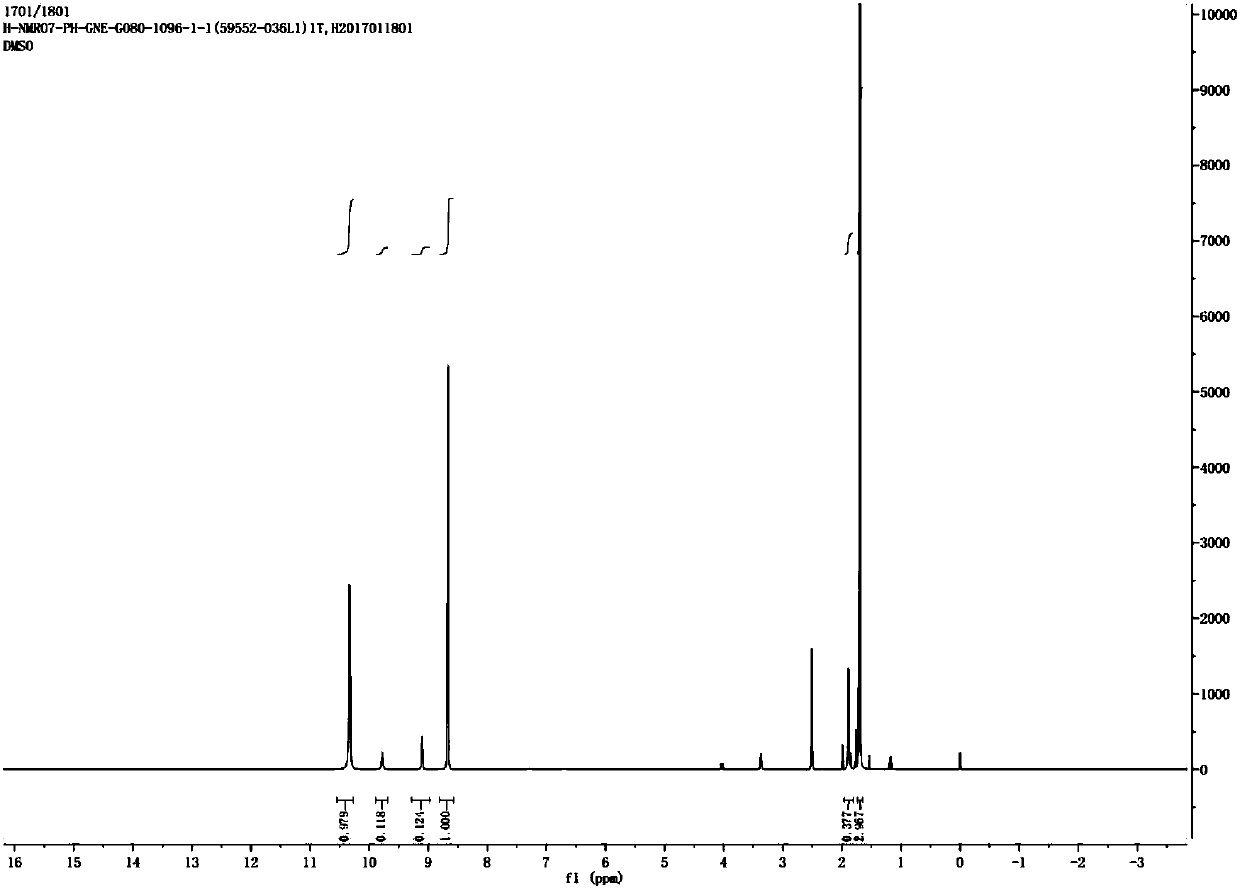
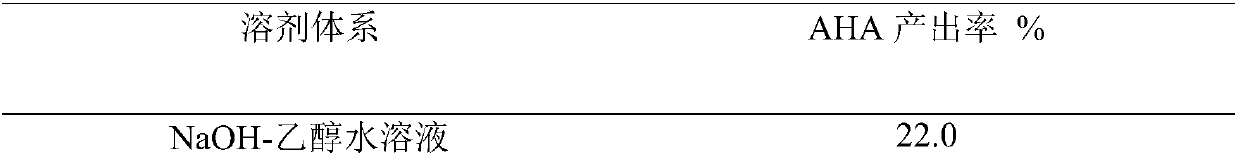
Synthesis method of urease inhibitor acetohydroxamic acid
A technology of acetohydroxamic acid and synthesis method, which is applied in the field of animal husbandry additive preparation, can solve problems such as unclear maximum output of products, and achieve high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Reaction system is that 1000g is standard with product final output: among the present embodiment, hydroxylamine hydrochloride consumption is 1300g (18.7mol), and ethyl acetate consumption is 1978g (86.3mmol), and the consumption of methyl alcohol is 2.6L, and the consumption of water is 2.6L, Sodium hydroxide consumption is 1496g (37.4mol); The proportioning of each composition: Hydroxylamine hydrochloride: Ethyl acetate: Water: Methanol: NaOH=1.0eq:1.2eq:2v:2v:2.0eq (wherein, eq is the molar weight ratio ratio; v is the volume ratio).
[0025] Reaction steps:
[0026] 1) Add 2.6L methanol and 1300g hydroxylamine hydrochloride successively to the three-necked reaction flask (10L), place the reaction flask in an ice-water bath, and keep stirring;
[0027] 2) Dissolve 748g of sodium hydroxide in 1.3L of water, then pour the sodium hydroxide solution into the reaction flask to neutralize the hydrochloric acid;
[0028] 3) Add 1978g of ethyl acetate into the reaction fla...
Embodiment 2I
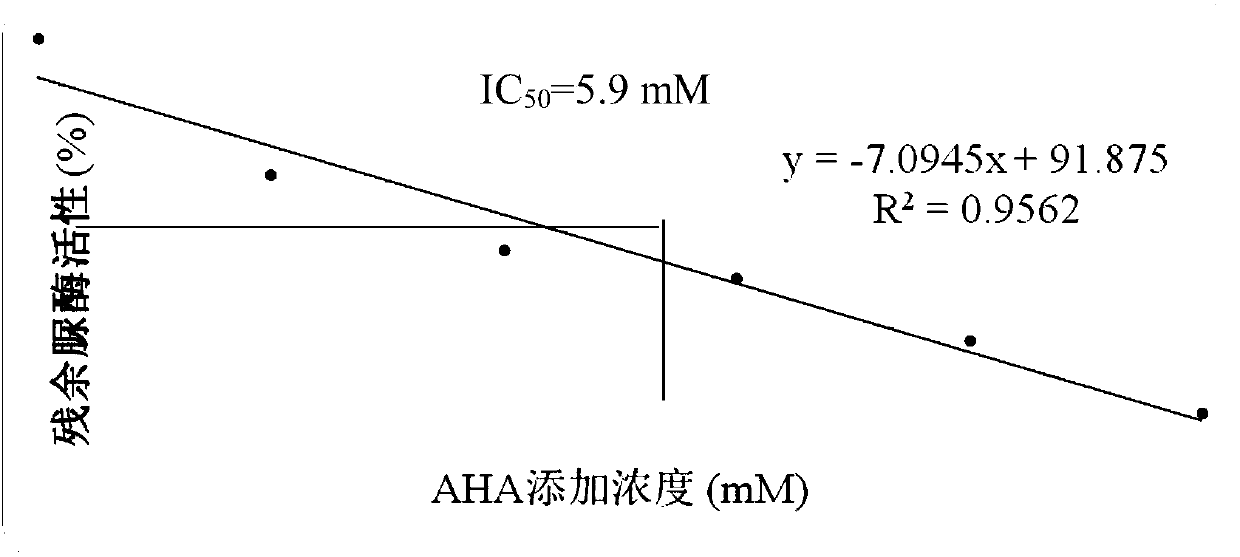
[0064] The mensuration of embodiment 2IC50 value
[0065] The inhibitory effect of the product acetohydroxamic acid on rumen urease activity in ruminants is expressed by IC50 value, that is, the concentration of acetohydroxamic acid that inhibits 50% of urease activity under certain reaction conditions. In this example, fresh rumen fluid was filtered through four layers of sterilized gauze, and the filtrate was subjected to differential centrifugation (500×g for 20 minutes and 12,000×g for 15 minutes) at 4° C. to obtain liquid-phase rumen microorganisms. Solid-phase rumen microorganisms were obtained after the feed residues were repeatedly washed twice with 50 mmol / l HEPES buffer (4-hydroxyethylpiperazineethanesulfonic acid) (pH 7.5). After mixing the solid and liquid phase microorganisms, add PBS (phosphate-buffered saline) (pH 7.5) buffer solution and ultrasonically break under low temperature conditions (40% strength, three times, 30s each time), and the supernatant is the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com