Method for whole-cell catalytic synthesis of 1-hydroxy-2-butanone
A whole-cell, hydroxyl-based technology with applications in bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Determination of formaldehyde lyase saturation mutation site
[0036] HotSpot Wizard 2.0 analyzes FLS mutation "hot spot" sites
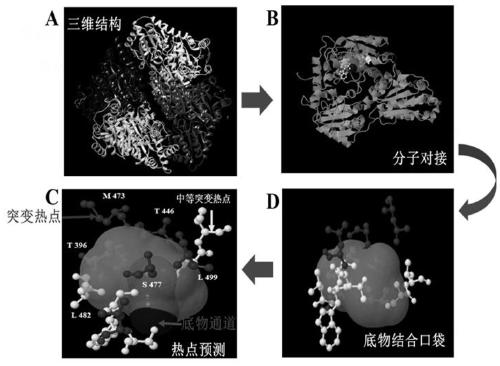
[0037]First download the formaldehyde lyase (FLS) protein structure pdb file with PDB number 4QPZ in the RCSB database, and use the HotSpot Wizard 2.0 server (http: / / loschmidt.chemi.muni.cz / hotspotwizard / ) to analyze the conservation of amino acids, Predict "hot spot" amino acid residues, find hot spot mutation sites around the substrate channel and active center, and then perform site-directed mutagenesis. The mutation site was identified as amino acid residue 482. The three-dimensional structure of amino acid residues predicted as "mutation hotspots" is shown in figure 1 .
Embodiment 2
[0038] Example 2 Construction of site-directed saturation mutation library
[0039] (1) PCR-based site-directed saturation mutation
[0040] The PCR-based site-directed saturation mutation method is to design degenerate primers at the determined mutation sites to amplify the target gene to obtain mutants. The codon corresponding to the degenerate primer was designed as NNK.
[0041] The specific steps are:
[0042] (1) The wild-type formaldehyde lyase gene (GenBank: AY007242.1) was screened in the NCBI database, and General (Anhui) Biological Company was commissioned to synthesize the gene, and cloned into the E. coli expression vector pET28a, and the recombinant plasmid was named is pET28a-FLS.
[0043] (2) By introducing a 15-25 bp homologous sequence at the end of the linearized vector at the 5' end of the primer, the 5' and 3' ends of the PCR product of the insert fragment have completely identical sequences corresponding to the two ends of the linearized vector Accord...
Embodiment 3
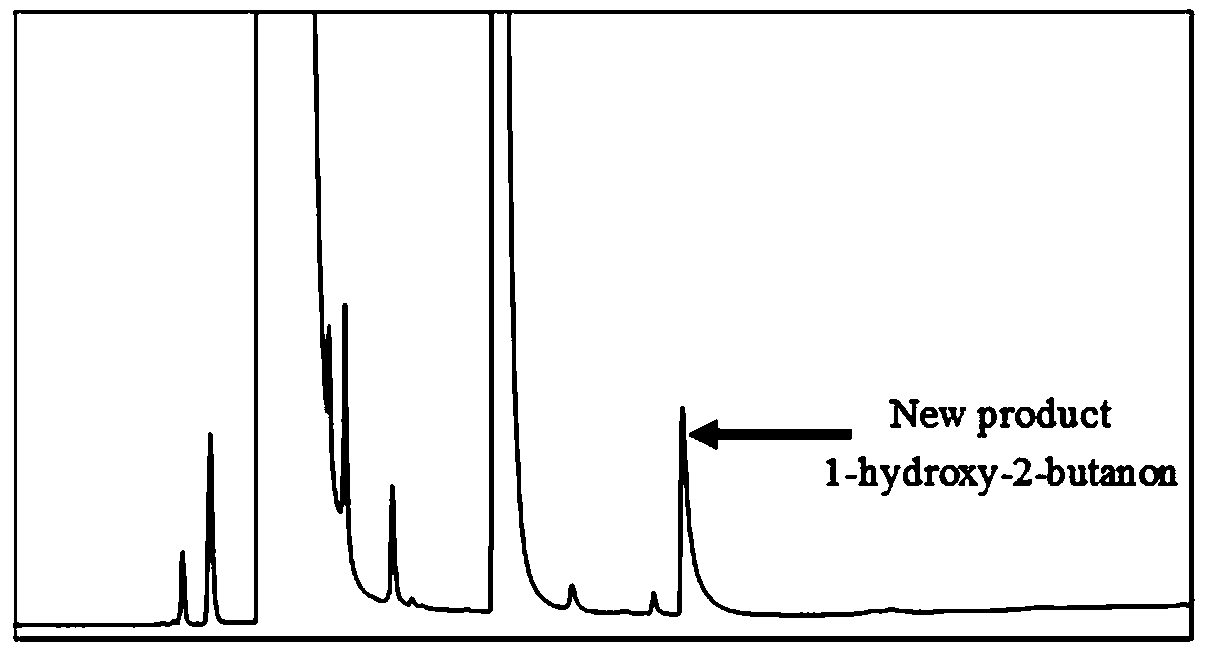
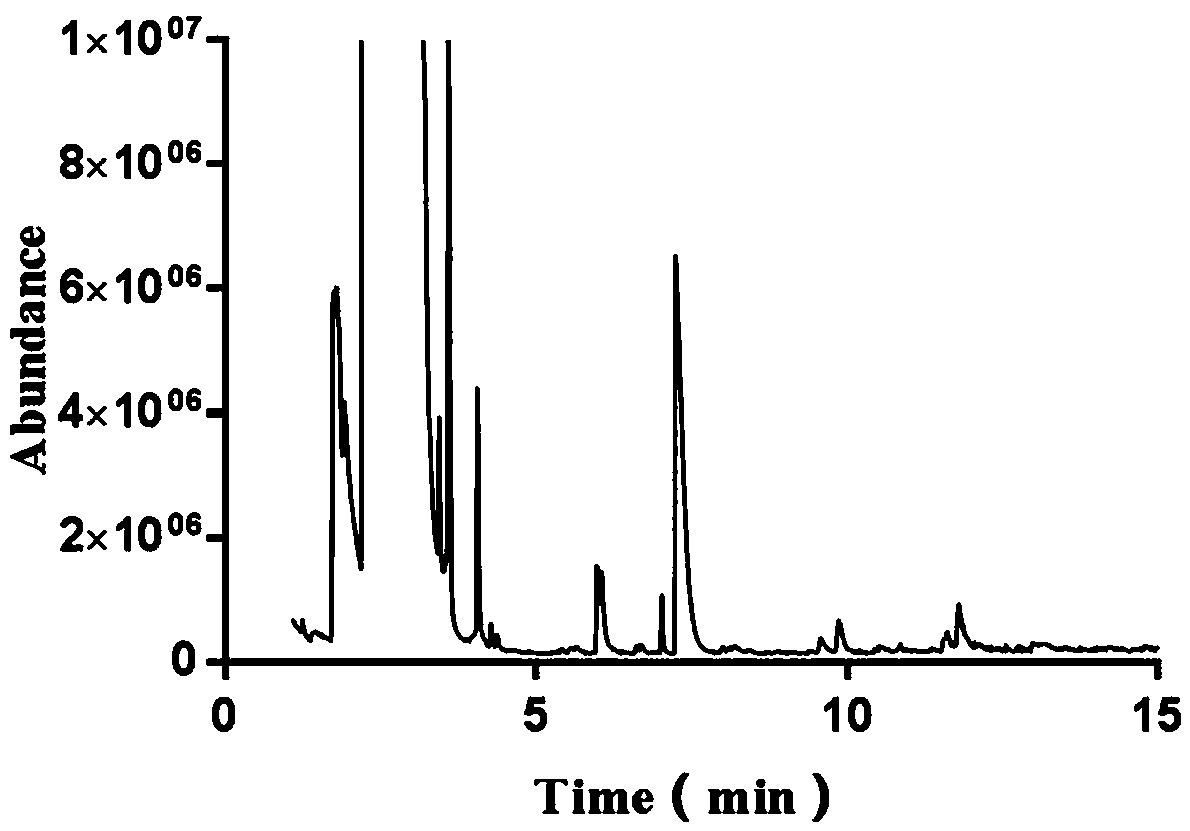
[0053] Example 3 Whole Cell Catalytic Synthesis of 1-Hydroxy-2 Butanone
[0054] The preparation method of the whole cells is as follows: inoculate the high-yielding beneficial mutant strains in fresh LB medium with kanamycin sulfate (Kan), cultivate overnight at 37°C and 180 rpm, and inoculate at 1% (v / v) The amount was transferred to 50mL LB liquid medium with kanamycin sulfate (Kan), placed in a shaker at 37°C, and cultivated to OD at 180 rpm 600 0.6-0.9, add IPTG at a final concentration of 0.5mmol / L, place on a shaker at 18°C and 180 rpm to induce expression for 24 hours. After the induced expression was completed, the cells were collected, centrifuged (4°C, 5000 rpm, 5 min), and washed (the cells were resuspended twice in 0.85% pre-cooled saline) as whole cells.
[0055] The whole-cell catalytic conditions are: 1 mL of catalytic system in a 1.5 mL centrifuge tube, 100 mM potassium phosphate buffer (pH=8), 0.1 mM TPP, 0.1 mM MgSO 4 , each concentration of substrate fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com