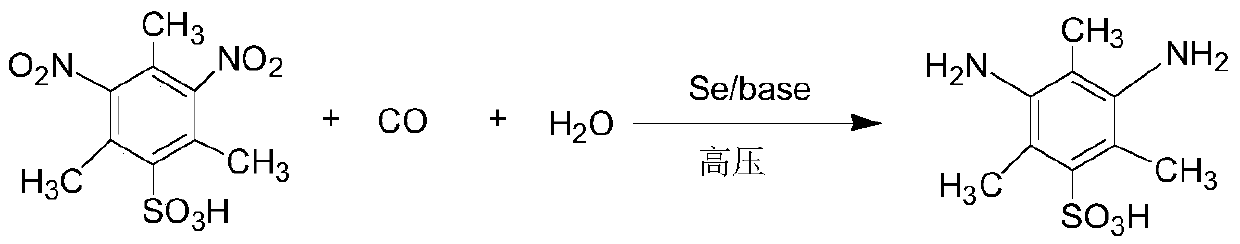
Method for synthesizing M acid
A technology of carbon monoxide and trimethylbenzenesulfonic acid, which is applied in chemical instruments and methods, sulfonic acid preparation, organic chemistry, etc., can solve the problems of easy deactivation of catalysts, influence of reaction process, increase of catalyst cost investment, etc., to avoid The effect of high three wastes, simple operation, easy separation, recycling and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take a 10L autoclave for washing and dry it for later use. Add 5-dinitro-2,4,6-trimethylbenzenesulfonic acid (0.5mol), inorganic base anhydrous sodium carbonate (0.6mol), selenium (0.025mol), and deionized water 3L into the autoclave in turn, Introduce carbon monoxide gas to 5MPa, react at 150°C for 5 hours, stir, filter, then adjust the pH to 1 with hydrochloric acid to precipitate the product, filter with suction, and recrystallize to obtain the pure product. The highest purity of the product measured by high performance liquid chromatography is 99.9%.
Embodiment 2
[0028] Other experimental methods and conditions are with embodiment 1, only change the reaction time, record the yield of M acid as table 1:
[0029] Table 1
[0030] time (h) 2 3 4 5 6 7 Yield (%) 53.94 69.4 76.7 84.21 78.24 75.22
Embodiment 3
[0032] Other experimental method and condition are with embodiment 1, only change the kind of alkali, record the yield of M acid as table 2:
[0033] Table 2
[0034] alkali Et 3 N
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com